Polylysine oligomer modified recombined apoferritin nanometer cage and preparation thereof
A technology of oligolysine and apoferritin, which is applied in the field of recombinant apoferritin nanocages and its preparation, can solve the problems of drug degradation and protein cage structure damage, reduce toxic side effects, increase concentration, and improve treatment effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1. Construction of pET-30a(+) / n-Lys-FTH expression engineering bacteria
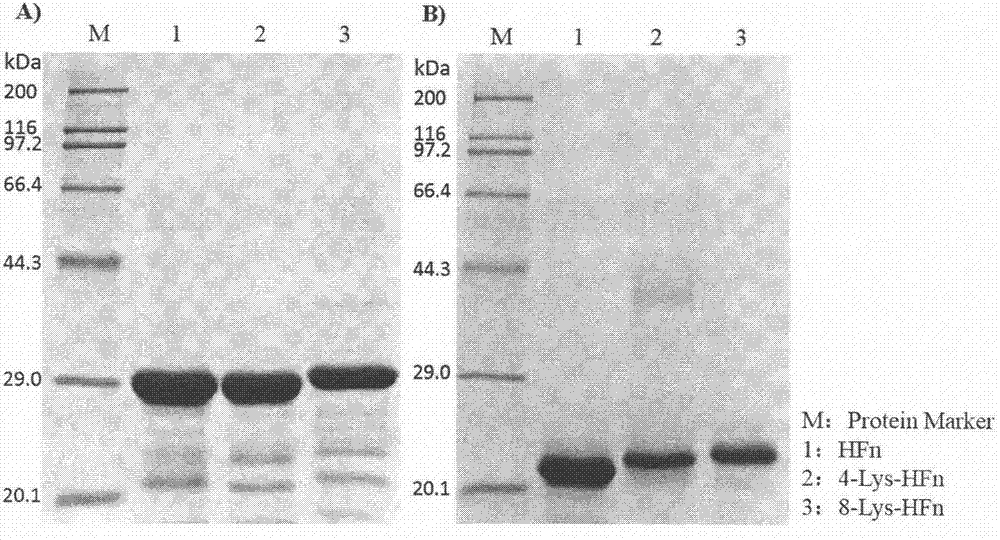
[0020] According to the FTH gene coding sequence to be fully synthesized as reported in the literature, in order to modify the N-terminal of the FTH subunit with different numbers of Lys, add AAGAAAAAGAAA (4-Lys) and AAGAAAAAGAAAAAGAAAAAGAAA (8- Lys), then add CCATGGCT and GCGGCCGC respectively to the 5' end and 3' end of the modified gene coding sequence to obtain Nco I and Not I restriction enzyme recognition sites, and finally the gene sequence is optimized so that the sequence is in Correct expression can be achieved in the E. coli system. The lengths of the two sequences are 580bp and 592bp respectively, and the nucleotide sequences encoding the 4-Lys-HFn and 8-Lys-HFn protein subunits are respectively shown in SEQ ID NO.1 and SEQ ID NO.2. The synthesized sequence was digested with Nco I and Not I, and the plasmid vector pET-30a(+) was subjected to the same double digestion, and t...
Embodiment 2
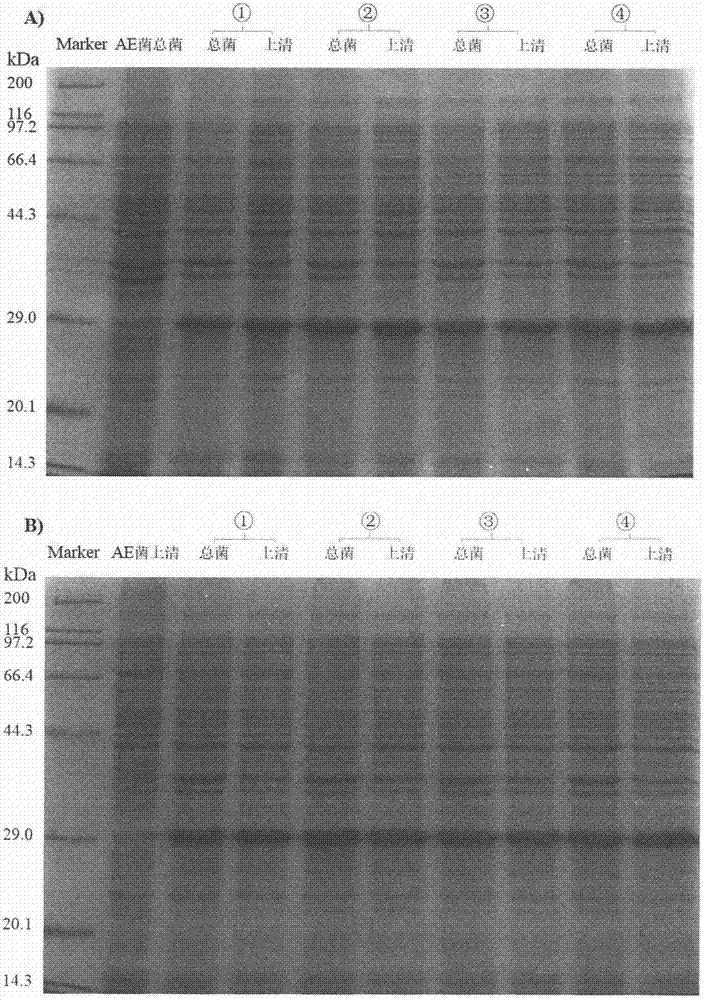
[0021] Example 2. Analysis of the soluble expression form of the fusion protein of n-Lys-HFn
[0022] Add positive recombinant bacteria to fresh Kan + - In LB medium, at 37°C, 220r / min, shake overnight to activate. Transfer to 5mL Kan at 1% inoculum size + -LB medium, at 37°C, 220r / min, cultured to the OD of the bacterial solution 600 reach 0.5. Add IPTG with a final concentration of 0.1 mM into the test tube, and induce expression for about 6 hours under the same conditions. Take 1mL of bacterial liquid and centrifuge at 8000×g for 3min at 4°C. After discarding the supernatant, add 200 μL of binding buffer (20 mM Tris, 5 mM imidazole, 0.5 M NaCl, pH7.9) to resuspend, resuspend and centrifuge again under the same conditions, discard the supernatant to collect bacteria, and resuspend in 200 μL of binding buffer, frozen overnight at -20°C. After the bacteria were taken out and melted, the bacteria were ultrasonically broken, and the sample was taken out as the total bacter...
Embodiment 3
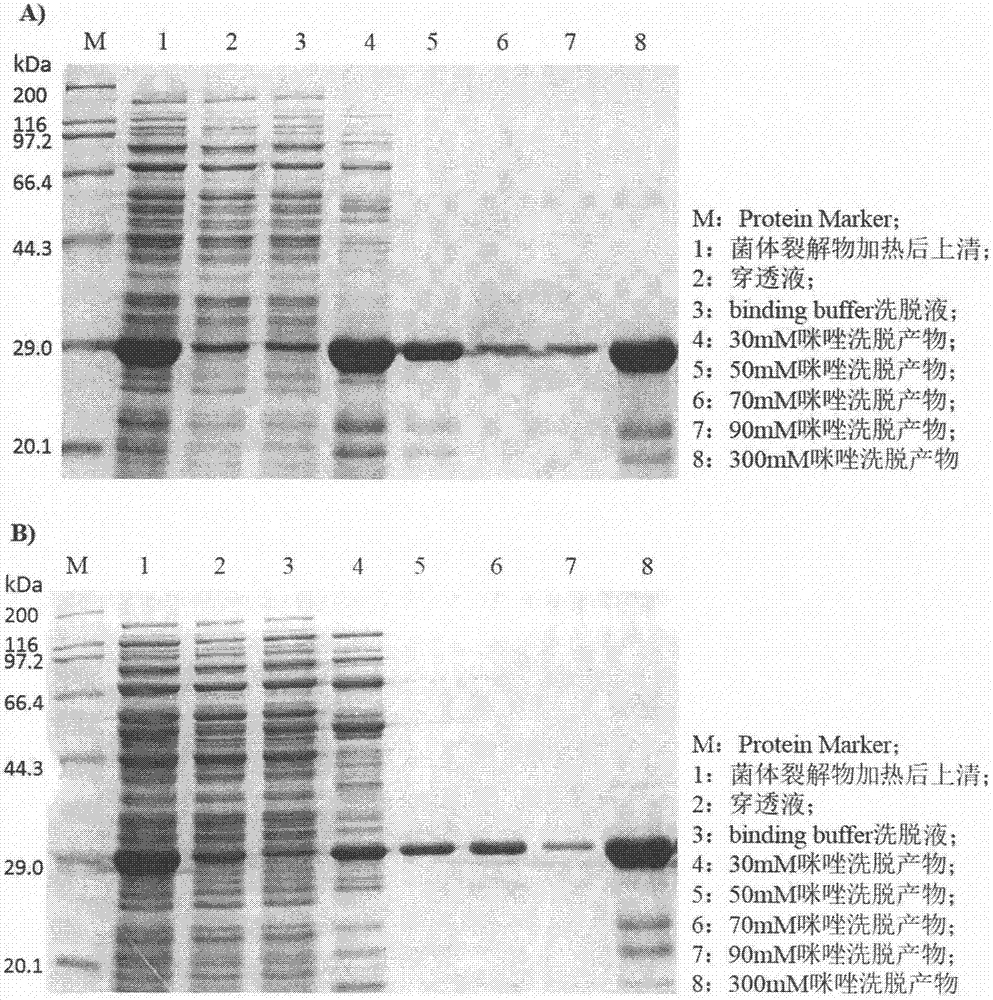
[0023] A large amount of expression and collection of bacteria of embodiment 3.n-Lys-HFn target fusion protein
[0024] Transfer the positive recombinant bacteria recovered overnight to 100mL Kan + -LB medium, at 37°C, 220r / min, cultured to the OD of the bacterial solution 600reach 0.5. Add IPTG with a final concentration of 0.1 mM into the test tube, and induce expression for about 6 hours under the same conditions. 4°C, 8000×g, centrifuge for 3 minutes to collect the bacteria, add 30mL of binding buffer to resuspend, resuspend and centrifuge again under the same conditions, discard the supernatant to collect the bacteria, resuspend in 30mL of binding buffer, add the final Lysozyme at a concentration of 100 μg / mL, frozen at -20°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com