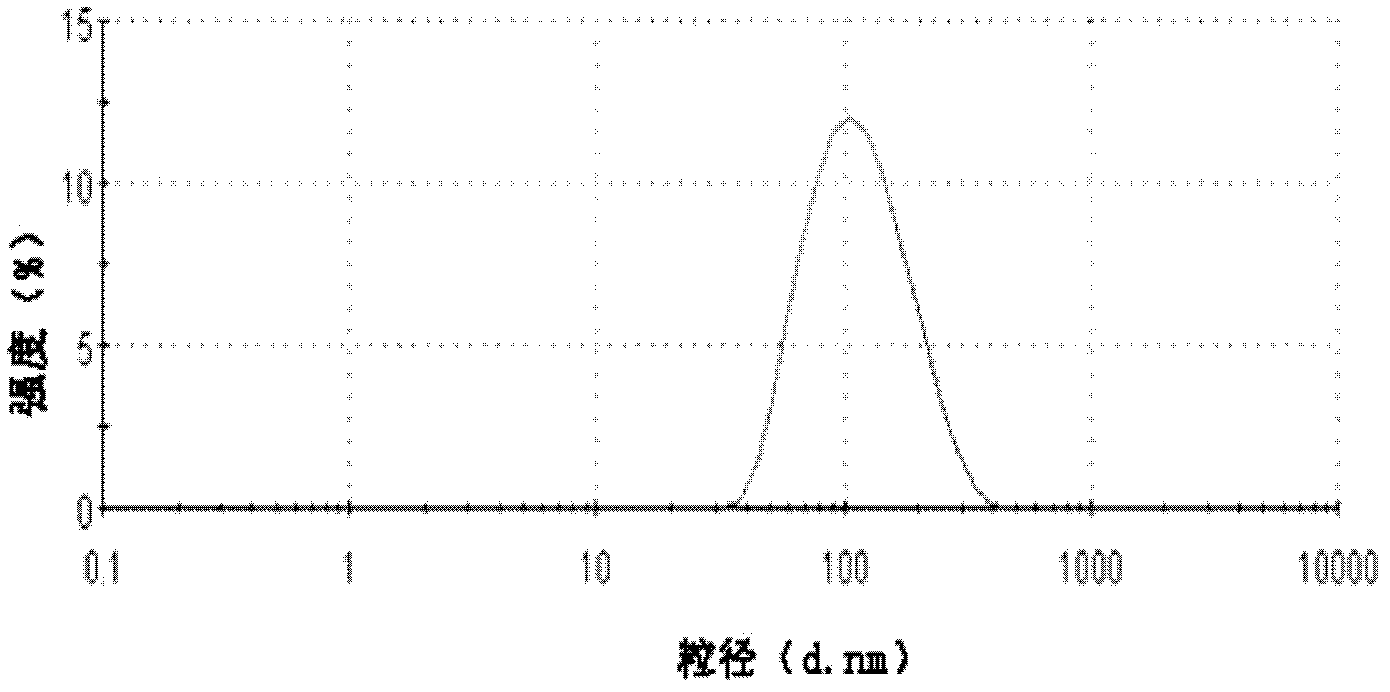
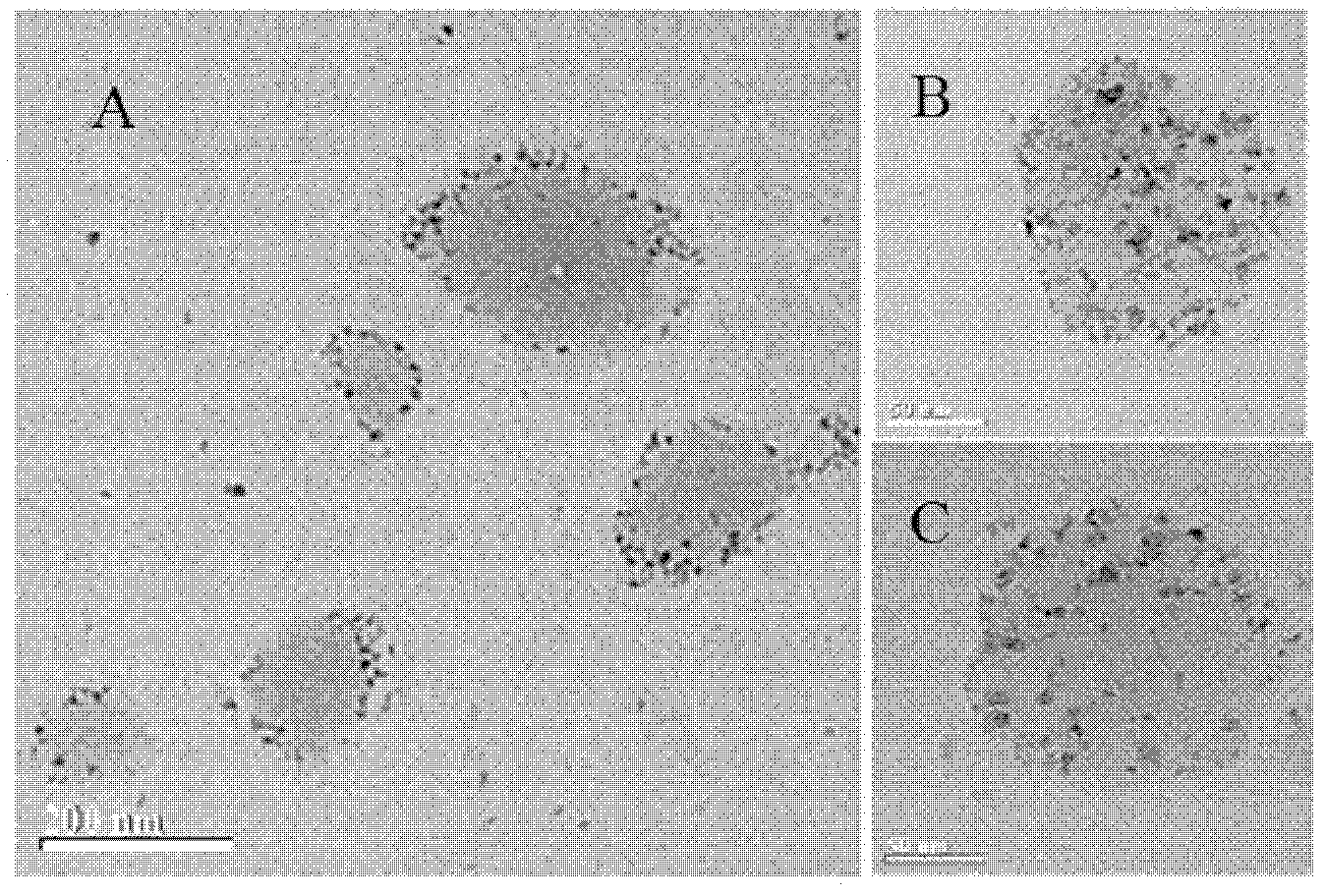
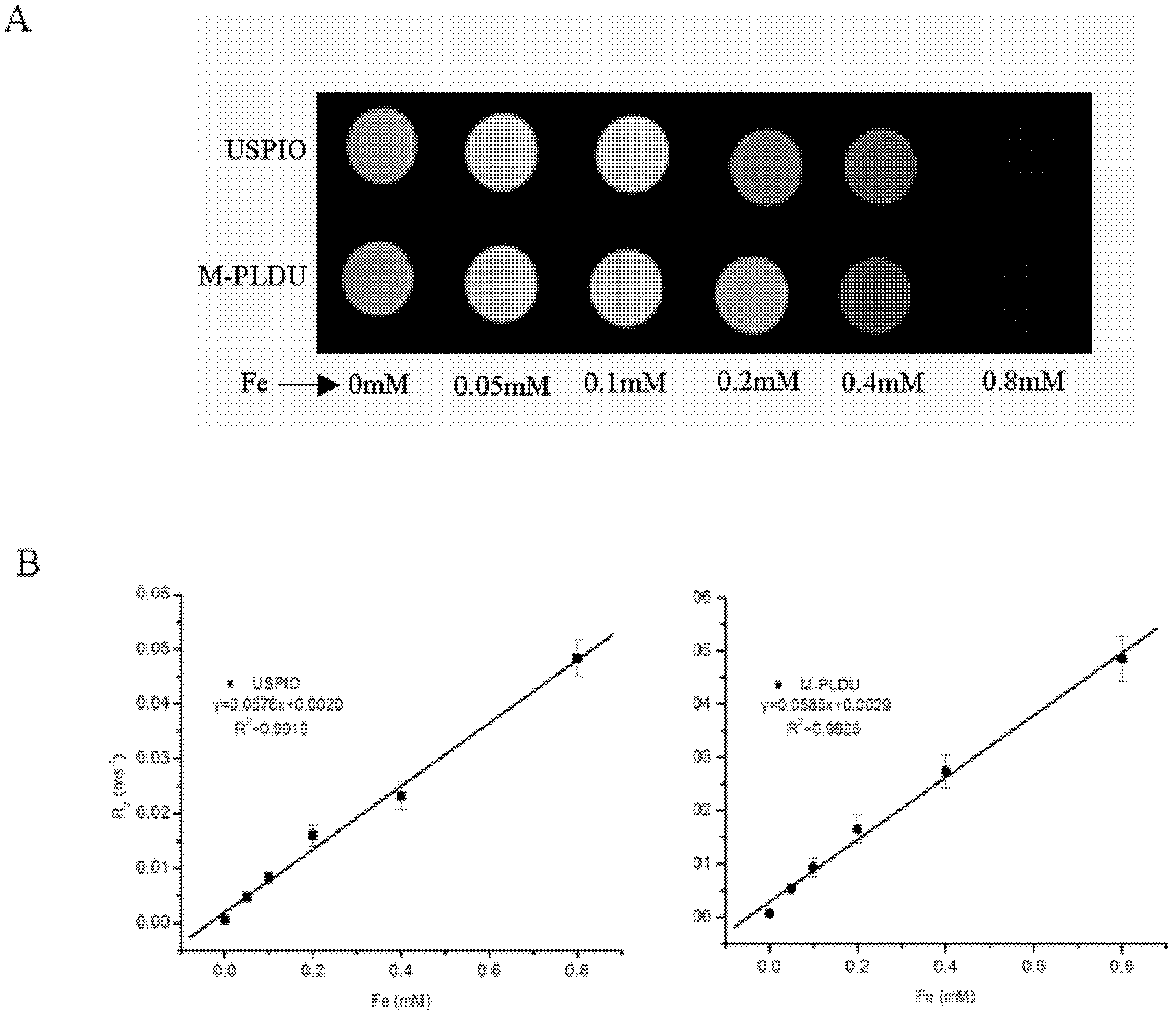
Invisible nano-liposome injection for encapsulating USPIO (Ultra-small Super Paramagnetic Iron Oxide) and preparation method and application thereof
A technology of nano-liposome and stealth liposome, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1: Adriamycin Hydrochloride Stealth Liposome Intravenous Injection Encapsulating USPIO
[0081] Composed of:
[0082]Soy lecithin 500mg, phosphatidylethanolamine 100mg, cholesterol 300mg, polyethylene glycol-distearoylphosphatidylethanolamine 100mg, USPIO 15mL, doxorubicin hydrochloride 100mg.
[0083] Preparation:
[0084] Take the USPIO solution and mix it with a phosphate buffer solution with pH=6, add 300 mg of sodium periodate, mix and oxidize for 30 min, add 200 mg of ethylene glycol to terminate the reaction, and pass through a Sephadex-G25 column for desalting and purification. Dissolve soybean lecithin, phosphatidylethanolamine, cholesterol, and polyethylene glycol-distearoylphosphatidylethanolamine in 30 mL of chloroform according to the above ratio, and put it in a rotary evaporator at 37°C for 40 minutes to remove the organic solvent to obtain lipid Plasma membrane; add USPIO solution to it and vortex for 20min, continue to add 20mL of phosphate...
Embodiment 2
[0085] Embodiment 2: Adriamycin Hydrochloride Stealth Liposome Intravenous Injection Encapsulating USPIO
[0086] Composed of:
[0087] Yolk lecithin 500mg, phosphatidylethanolamine 100mg, dioleoylphosphatidylethanolamine 100mg, cholesterol 400mg, polyethylene glycol-dipalmitoylphosphatidylcholine 150mg, USPIO 15mL, doxorubicin hydrochloride 100mg.
[0088] Preparation:
[0089] Take the USPIO solution and mix it with a phosphate buffer solution with pH=6, add 300 mg of sodium periodate, mix and oxidize for 30 min, add 200 mg of ethylene glycol to terminate the reaction, and pass through a Sephadex-G25 column for desalting and purification. Dissolve egg yolk lecithin, phosphatidylethanolamine, dioleoylphosphatidylethanolamine, cholesterol, polyethylene glycol-dipalmitoylphosphatidylcholine in 50mL of ether according to the above ratio, and put it in a rotary evaporator at 40°C. 30min, remove organic solvent to obtain lipid film; Add USPIO solution wherein and vortex 20min, c...
Embodiment 3
[0090] Embodiment 3: Adriamycin Hydrochloride Stealth Liposome Intravenous Injection Encapsulating USPIO
[0091] Composed of:
[0092] Egg yolk lecithin 300mg, hydrogenated soybean lecithin 300mg, dimyristoyl phosphatidylethanolamine 150mg, cholesterol 400mg, polyethylene glycol-polyglycolide lactide 150mg, USPIO 20mL, doxorubicin hydrochloride 100mg.
[0093] Preparation:
[0094] Take the USPIO solution and mix it with pH=6 phosphate buffer, add 300 mg of sodium periodate, mix and oxidize for 40 min, add 300 mg of ethylene glycol to terminate the reaction, and pass through a Sephadex-G25 column for desalting and purification. Dissolve egg yolk lecithin, hydrogenated soybean lecithin, dimyristoyl phosphatidylethanolamine, cholesterol, polyethylene glycol-polyglycolide lactide in 30 mL of chloroform according to the above ratio, and put it in a rotary evaporator at 37 °C Rotary steam for 40 minutes, remove the organic solvent to obtain a lipid film; add USPIO solution to it...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com