Preparation method of wholly-inorganic lead-containing halide perovskite quantum dot fluorescent powder
A halide perovskite and quantum dot technology, which is applied in chemical instruments and methods, luminescent materials, nanotechnology, etc., can solve the problem of poor water and thermal stability of perovskite quantum dots, affecting the application performance of perovskite quantum dots and Foreground, quantum dot water and thermal stability can not be satisfied and other problems, to achieve the effect of high repetition rate, short reaction time, strong water stability and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
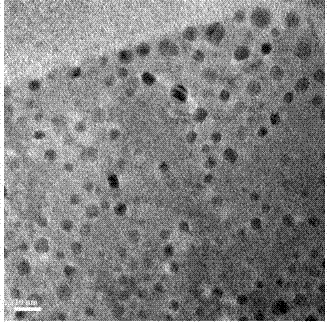
[0033] In this example, the dissolution-precipitation method is used to synthesize all-inorganic lead-containing halide quantum dots at room temperature, which specifically includes the following steps:
[0034] (1) Metal halide salts CsBr and PbBr 2 According to the molar ratio of 1:1, add in the DMF solution, under the magnetic stirring of 1000r / min until the metal halide salts CsBr and PbBr 2 completely dissolved.
[0035] (2) To the DMF solution of the metal halide salt obtained in (1), add 150 mg (10 mg / mL) of tetradecyl phosphoric acid and 1 mL of oleylamine as surfactants, and tetradecyl phosphonic acid as Precursors of organophosphates. Under magnetic stirring, a homogeneous mixed solution was formed as a reaction precursor.
[0036] (3) Add 1 mL of the precursor solution to the rapidly stirred 10 mL toluene solution to generate a quantum dot reaction solution.
[0037] (4) Centrifuge the above quantum dot reaction solution, pour the supernatant, and redisperse the...
Embodiment 2
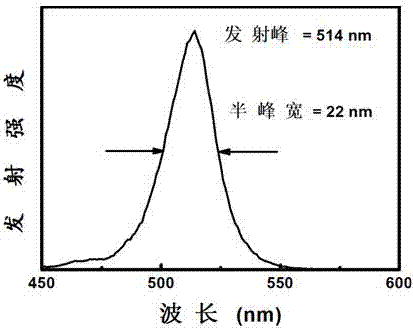
[0039] Based on implementation case 1, the CsPbBr prepared by implementation case 1 3 The quantum dot reaction solution was washed by centrifugation for 3 times, and the precipitation of quantum dots was kept in the air at 35°C for 48 hours to form CsPbBr through self-assembly 3 Composite phosphor of quantum dots / tetradecyl phosphate.
[0040] Referring to Example 2, the difference is that the amount of all raw materials used is 4 times that of Example 1, so as to mass produce quantum dot phosphors. Finally, 1.5 g of CsPbBr was obtained at one time 3 Composite phosphor of quantum dots / tetradecyl phosphate.
Embodiment 3
[0042] Similar to Example 2, the difference is that the CsBr and PbBr in Example 2 2 Molar ratio 1:1 changed to CsI and PbI 2 Molar ratio 1:1, you can get CsPbI 3 Composite phosphor of quantum dots / tetradecyl phosphate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com