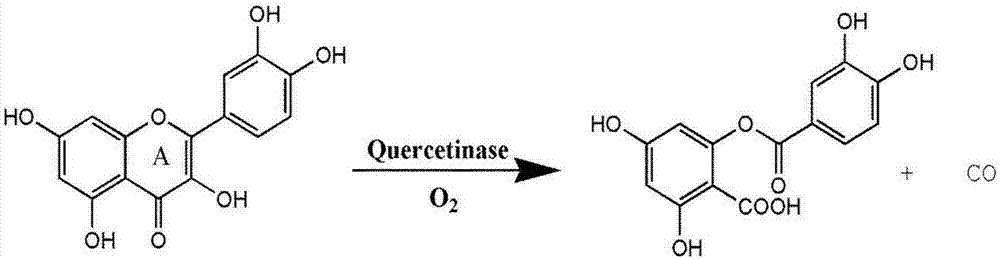
Light induced carbon monoxide release molecule and preparation method thereof
A carbon monoxide and light-induced technology is applied to zinc organic compounds, 2/12 group organic compounds without C-metal bonds, anti-toxic agents, etc. It can solve the problems of short irradiation wavelength, unfavorable application, and restricted development, etc., and achieve process can be achieved control, good CO release performance, and reduced side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
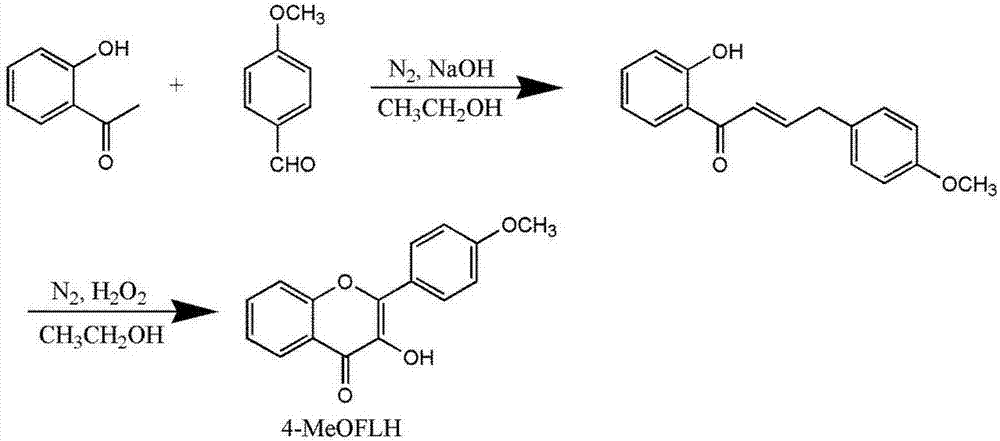
[0041] Step 1. Preparation of CO release unit
[0042] 4-methoxyflavonol (4-MeOFLH) was synthesized from p-methoxybenzaldehyde and 2-hydroxyacetophenone as starting materials, and the product was used as CO releasing unit.
[0043] refer to figure 2 , the method for synthesizing 4-methoxy flavonol is as follows:
[0044] in N 2 Under protection, p-methoxybenzaldehyde (0.12 mL, 1 mmol) was dissolved in 10 mL of ethanol, and 1 mL of 25% NaOH aqueous solution was added to obtain a colorless mixed solution. A solution of 2-hydroxyacetophenone (0.12 mL, 1 mmol) in ethanol (3 mL) was added to the above colorless mixed solution, and the reaction was continued for 5 h. Cool the reactant in an ice bath, add dropwise 30% acetic acid solution to adjust the pH to weak acidity, continue stirring for 30 min, filter, collect the precipitate, and dry to give chalcone dark yellow powder (0.16g, 61.3%, Mp: 91°C) . The obtained chalcone (0.16 g, 0.62 mmol) was dissolved in 5 mL of absolute...
Embodiment 2
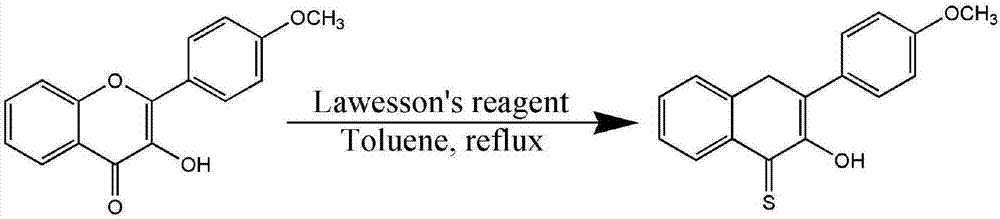
[0073] Step 1. Preparation of CO release unit
[0074] 4-Methoxyflavanol (4-MeOFLTH) was synthesized from p-methoxybenzaldehyde and 2-hydroxyacetophenone as starting materials, and the product was used as CO releasing unit.
[0075] The synthesis process is divided into two steps:
[0076] (1) Using p-methoxybenzaldehyde and 2-hydroxyacetophenone as starting materials to first synthesize 4-methoxyflavonol, this step is exactly the same as step 1 in Example 1, and will not be repeated;
[0077] (2) The 4-methoxyflavonol synthesized in the previous step is used as a reactant to synthesize 4-methoxyflavonol, and the synthesis process will be described in detail below.
[0078] refer to image 3 , the method for synthesizing 4-methoxyl thioketol is as follows:
[0079] in N 2 Under protection, dissolve 4-methoxyflavonol (1.01g, 3.78mmol) in 120mL toluene, stir at room temperature for 5min, add Lawson's reagent (1.2mg, 3.03mmol), reflux for 30min, the solution turns dark red, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com