Preparation and evaluation of novel RGDF-targeting ruthenium polypyridine coordination compound vector
A bipyridine and targeting technology, which is applied in the field of preparation and evaluation of new ruthenium polypyridine complex carriers targeted by RGDF, can solve the problems of cells that cannot pass through the cell membrane
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] The preparation of embodiment 1.Ru-CO-RGDF
[0103] Ru-CO-RGDF preparation route such as figure 1
[0104] Synthesis of Boc-Arg(Tos)-Gly-Asp(OBzl)-Phe-OBzl
[0105] The main chain of arginine and aspartic acid is protected by Boc at the amino terminus, the carboxyl terminus of glycine and benzoic acid is protected by OBzl, and the side chain is protected by a suitable protecting group (tert-butoxycarbonyl) L-amino acid, liquid phase method The peptide was connected to obtain two fully protected dipeptides: Boc-Arg(Tos)-Gly-OBzl and Boc-Asp(OBzl)-Phe-OBzl. After removing the protection of OBzl by hydrogenolysis and the protection of Boc by acidolysis, respectively, the peptide was connected with the liquid phase method to obtain the fully protected tetrapeptide. Under the condition of ice bath, dissolve 170 mg of Boc-Arg(Tos)-Gly-Asp(OBzl)-Phe-OBzl with 5 ml of HCl-dissolved ethyl acetate solution, stir the reaction, and monitor the reaction with a TLC plate. After t...
example 2
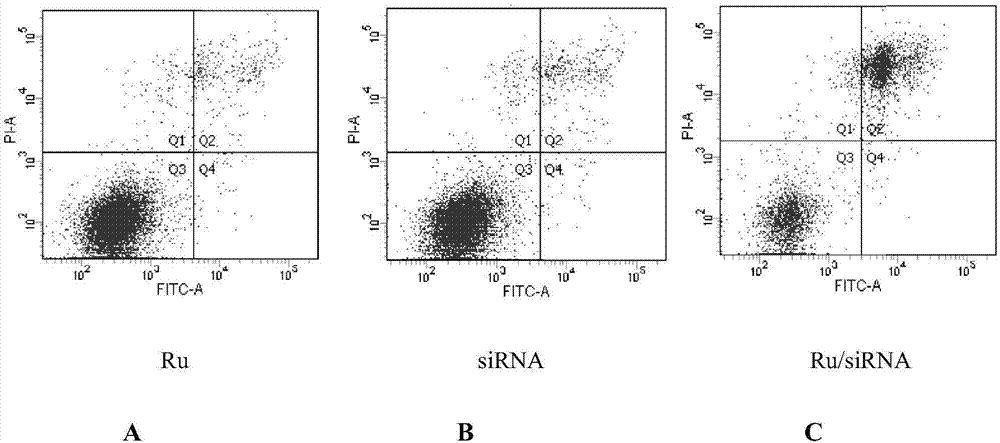
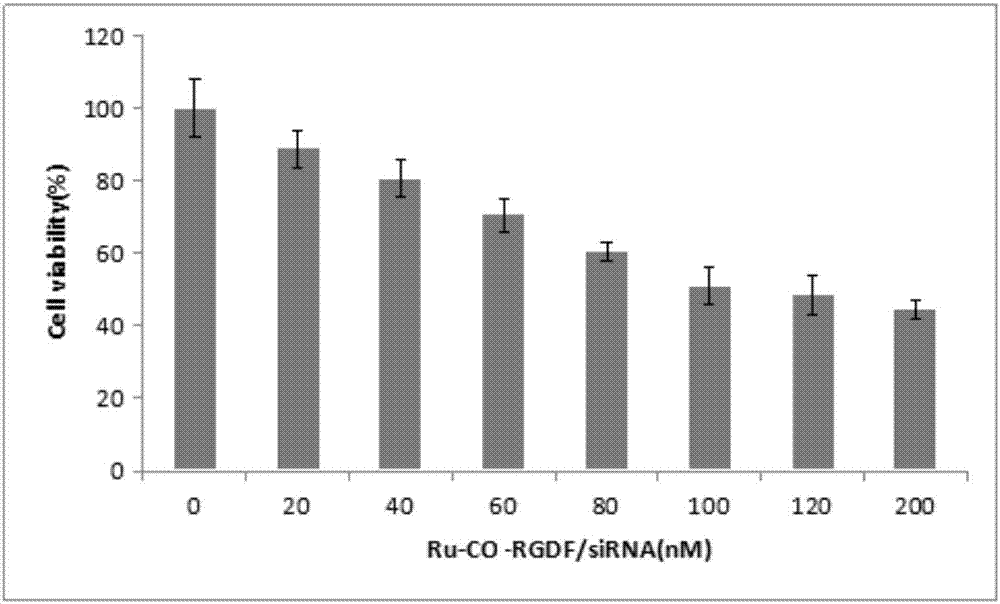
[0116] Example 2 Determination of the loading capacity of survivin-siRNA in Ru-CO-RGDF / survivin-siRNA
[0117] Prepare samples
[0118] Use RNA dilution buffer to prepare different concentrations of Ru-CO-RGDF 2, 1, 0.5, 0.25, 0.125, 0.0625, 0.03125 mg / mL, and different ratios of protamine mixed solutions, mix well, and stand at room temperature for 10 minutes , were mixed with 2 μL of survivin-siRNA, beat repeatedly, and stood for 10 minutes. Then add an appropriate volume of 5×Loading Buffer, vortex and mix well, and add 20 μL of the above solution to the prepared agarose gel sample well with a protein sample pipette tip.
[0119] Experimental procedure
[0120] Weigh 0.5g of agarose into a 100mL beaker, add 5mL of TBE (10×) and 45mL of deionized water, heat in a microwave oven on medium-high heat for 2min to dissolve the agarose. After cooling down slightly, add 8 μL of ethidium bromide solution (10 mg / mL), stir well, pour into the mold (washed in advance and rinse with ...
Embodiment 2
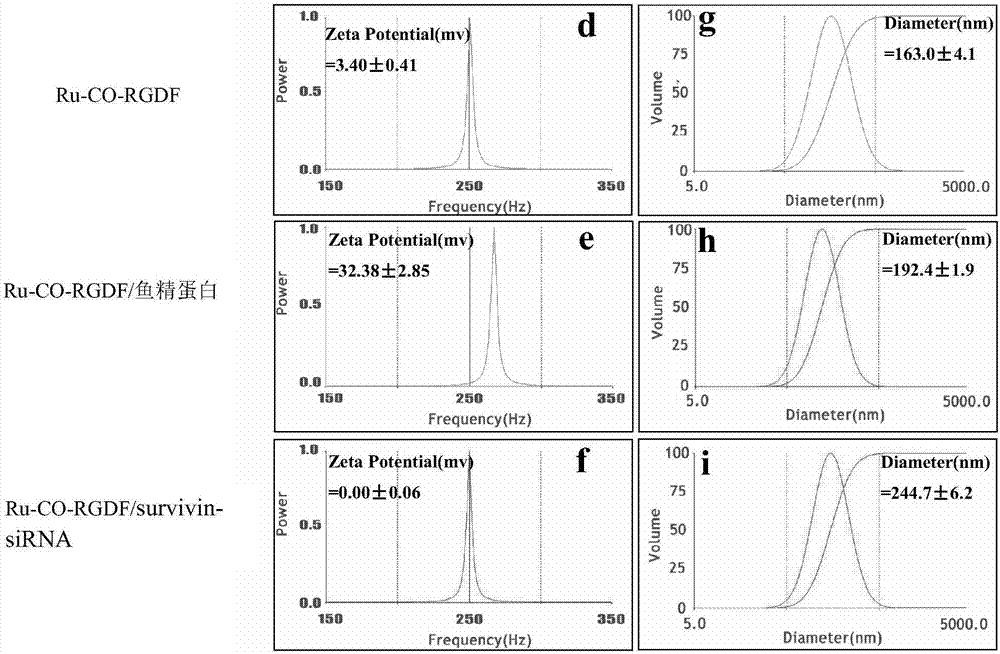
[0123] Example 2 Determination of the nanostructure of Ru-CO-RGDF and Ru-CO-RGDF / survivin-siRNA
[0124] experimental method
[0125] Take 10 μL of Ru-CO-RGDF aqueous dispersion with a concentration of 625 μg / mL, prepare Ru-CO-RGDF / protamine aqueous dispersion according to the volume ratio of 1:8, then mix 2 μL of siRNA and appropriate calf thymus DNA, take 20 μL of loaded survivin-siRNA and unloaded pure carrier were dropped onto the carbon support membrane (copper grid), and dried in an oven at 37°C. Each sample was observed under a transmission electron microscope (TEM, JEM-1230, JEOL, Japan) and photographs were taken. Then 20 μL of each was dropped onto a glass slide, and left to air dry at room temperature. Each sample was observed under a scanning electron microscope (JSM-6360LV, JEOL, JAPAN) and photographs were taken.
[0126] Experimental results
[0127] from Figure 11 From the scanning electron microscope and transmission electron microscope images, we can se...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com