Novel racemized chiral organic boron-nitride fluorescent compound and preparation method thereof
A fluorescent compound and a racemic technology, which is applied in the field of novel quinoline chiral organoboron nitrogen compounds and their preparation, can solve the problems of unreported synthesis of chiral quinoline boron nitrogen compounds and the like.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
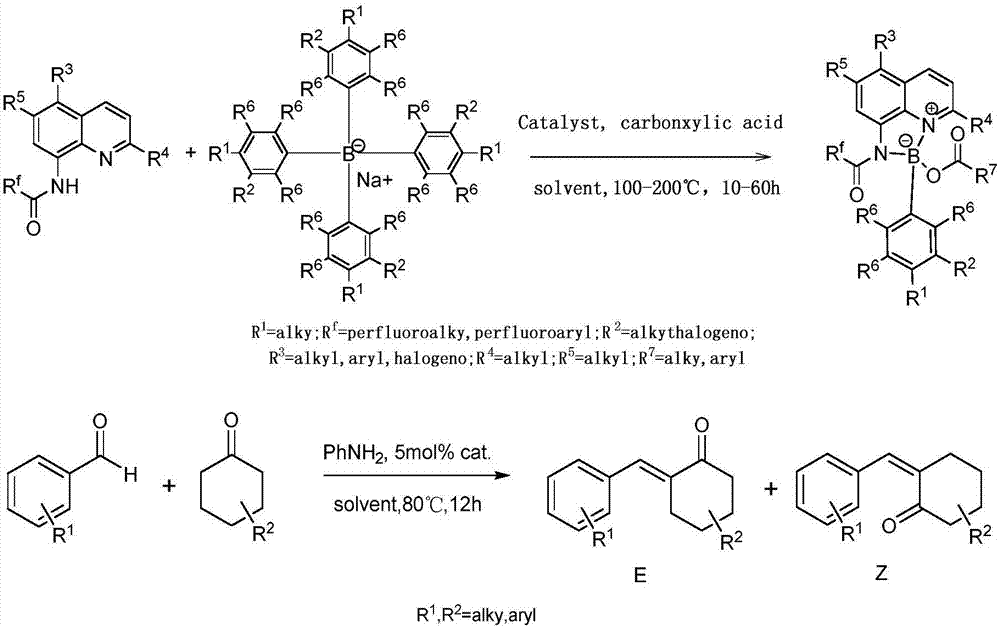
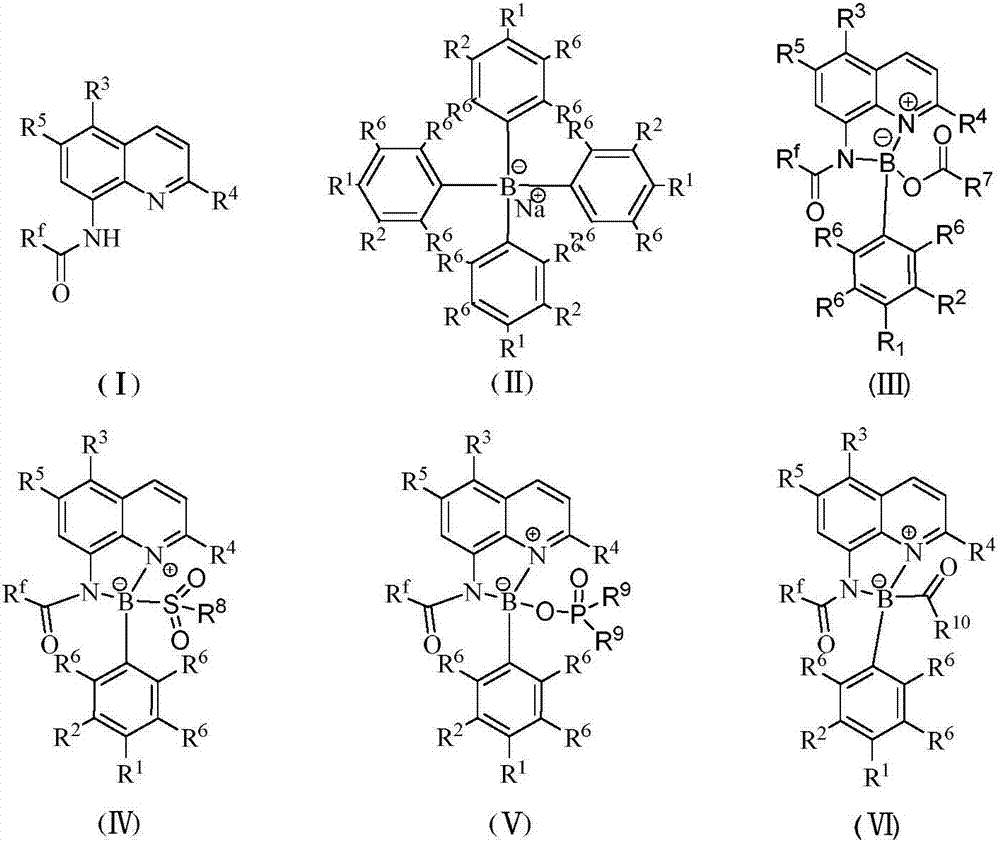
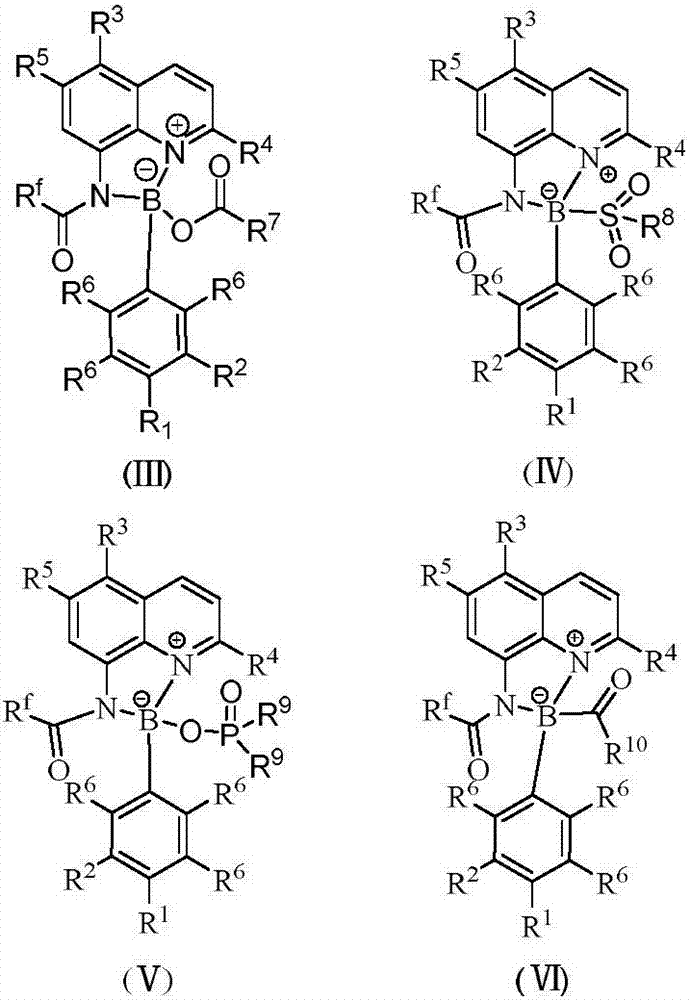
[0025] Add 0.1 mmol I (wherein R f = CF 3 ; 3 , R 4 , R 5 =H), 0.1mmol II (wherein R 1 , R 2 , R 6 = H), 0.005 mmol CuI, 0.1 mmol R 7 COOH(R 7 =Ph) and 1mL touluene, under nitrogen atmosphere, the reaction was carried out at 150°C for 12h. After the reaction was completed, filtered, concentrated, and separated by chromatography to obtain III (wherein R f = CF 3 ; 1 , R 2 , R 3 , R 4 , R 5 , R 6 = H, R 7 =Ph), the productive rate is 81%. Detection of catalytic activity: add 0.5mmol aniline (Ar=Ph), 0.5mmol benzaldehyde (Ar=Ph), 0.6mmol4-phenylcyclohexanone, 0.002mmol III (where R f = CF 3 ; 1 , R 2 , R 3 , R 4 , R 5 , R 6 = H, R 7 =Ph) and 1mL toluene, the reaction was carried out at 80°C for 12h, and TLC followed the reaction until the reaction was complete. The reaction result was: (E)-2-benzyl-4-phenylcyclohexanone, and the yield was 95%, ( The selectivity of E)-2-benzyl-4-phenylcyclohexanone is 100%, and the cis-trans selectivity is 1 / 99.
preparation example 2
[0027]Add 0.1 mmol I (wherein R f = CF 3 ; 3 , R 4 , R 5 =H), 0.1mmol II (wherein R 1 , R 2 , R 6 = H), 0.005 mmol KI, 0.1 mmol R 7 COOH(R 7 =Ph) and 1mL touluene, under nitrogen atmosphere, the reaction was carried out at 150°C for 12h. After the reaction was completed, filtered, concentrated, and separated by chromatography to obtain III (wherein R f = CF 3 ; 1 , R 2 , R 3 , R 4 , R 5 , R 6 = H, R 7 =Ph), the yield is 83%. Detection of catalytic activity: add 0.5mmol aniline (Ar=Ph), 0.5mmol benzaldehyde (Ar=Ph), 0.6mmol4-methoxycyclohexanone, 0.002 mmol III (where R f = CF 3 ; 1 , R 2 , R 3 , R 4 , R 5 , R 6 = H, R 7 =Ph) and 1mL toluene, the reaction was carried out at 80°C for 12h, TLC followed the reaction until the reaction was complete. The reaction result was: (E)-2-benzyl-4-methoxycyclohexanone, the yield was 97%, The selectivity of (E)-2-benzyl-4-methoxycyclohexanone was 100%, and the cis-trans selectivity was 1 / 99.
preparation example 3
[0029] Add 0.1 mmol I (wherein R f = CF 3 ; 3 , R 4 , R 5 =H), 0.1mmol II (wherein R 1 , R 2 , R 6 = H), 0.005 mmol LiI, 0.1 mmol R 7 COOH(R 7 =Ph) and 1mL touluene, under nitrogen atmosphere, the reaction was carried out at 150°C for 12h. After the reaction was completed, filtered, concentrated, and separated by chromatography to obtain III (wherein R f = CF 3 ; 1 , R 2 , R 3 , R 4 , R 5 , R 6 = H, R 7 =Ph), the productive rate is 84%. Detection of catalytic activity: add 0.5mmol aniline (Ar=Ph), 0.5mmol benzaldehyde (Ar=Ph), 0.6mmol4-trifluoromethylcyclohexanone, 0.002mmol III (where R f = CF 3 ; 1 , R 2 , R 3 , R 4 , R 5 , R 6 = H, R 7 =Ph) and 1mL toluene, the reaction was carried out at 80°C for 12h, TLC followed the reaction until the reaction was complete. The reaction result was: (E)-2-benzyl-4-trifluoromethylcyclohexanone, the yield was 91% , The selectivity of (E)-2-benzyl-4-trifluoromethylcyclohexanone is 100%, and the cis-trans selectivity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com