Cosmetic composition for containing nicotinoyl peptide and fermented natural extracts
A technology of cosmetic composition and fermented product, which is applied in the direction of cosmetics, medical preparations containing active ingredients, cosmetic preparations, etc., which can solve the problems of non-functionality and easy deterioration of cosmetic compositions, and achieve excellent wrinkle improvement effect and smooth skin. Excellent whitening effect and increased skin improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
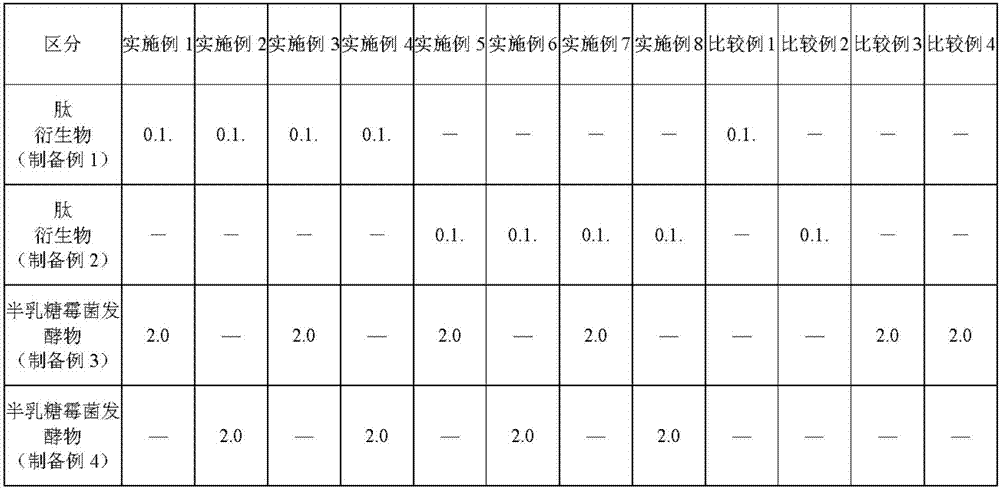
Examples
preparation example Construction
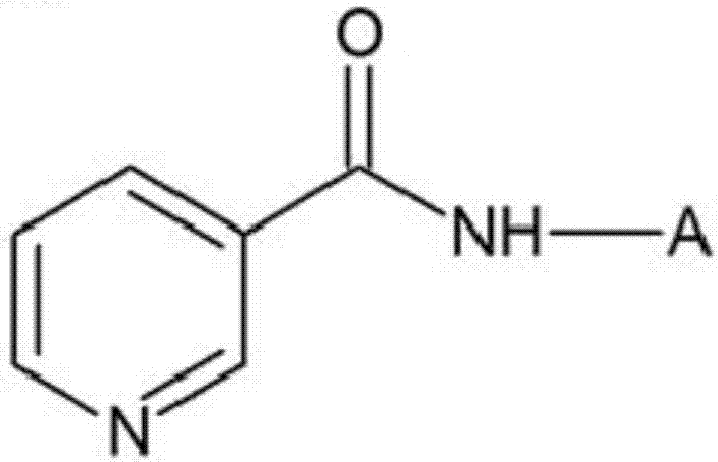
[0040] The preparation method of the above-mentioned oligopeptide is as follows: After extracting the protein in the living body, it is treated with protease to reduce its molecular weight, or it can be prepared biologically by using a gene recombination and protein expression system, or it can be prepared by using a peptide synthesizer, etc. Prepared by chemical synthesis.
[0041] Specifically, the above-mentioned oligopeptides can be prepared through the following steps, that is, step a, using the usual solid phase peptide synthesis (solid phase peptide synthesis, SPPS) to obtain NH 2 - protected peptide-resin; step b, making the acquired NH 2 - reacting the protected peptide-resin with niacin; and step c, removing the resin. In the case where there is a functional group in the side chain of the amino acid residue forming the above-mentioned oligopeptide, in the above-mentioned step a, the amino acid whose above-mentioned functional group is protected can be used to synthe...
preparation example 1
[0080] Preparation Example 1: Preparation of Nicotinyl Pentapeptide (YGGFM)
[0081] Pentapeptides are usually synthesized by using 9-fluorenylmethoxycarbonyl (9-fluorenylmethoxycarbonyl; Fmoc) as a protecting group for amino acids in solid-phase peptide synthesis, and N-hydroxybenzotriazole (N-hydroxybenzotriazole; HOBt ) and N, N-diisopropylcarbodiimide (N, N'-diisopropylcarbodiimide; DIC) were used as activators to extend amino acid residues (references: Wang C. Chan, Perter D. White, 'Fmoc- solid phase peptide synthesis', Oxford).
[0082] In the synthetic NH 2 - Add 20% piperidine / N-methylpyrrolidone solution to the protected peptide (YGGFM) resin to remove the 9-fluorenylmethoxycarbonyl group combined with the amino group, and use N-methylpyrrolidone (N-methyl-2 - After washing with pyrrolidone (NMP) and dichloromethane (dichloromethane: DCM), the coupling reaction was carried out with 5 equivalents of nicotinic acid (nicotinic acid) for 12 hours at room temperature. ...
preparation example 2
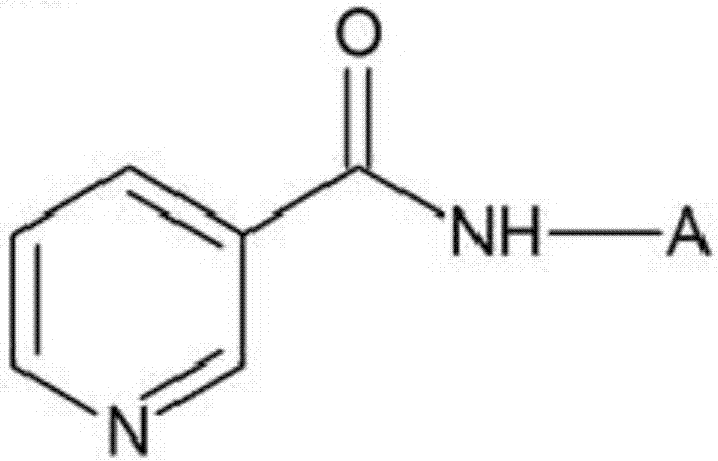
[0085] Preparation Example 2: Preparation of Nicotinyl-nonapeptide (YGGFLRKYP)
[0086] NH was synthesized according to the method described in Example 1 above 2 -Protected peptide (YGGFLRKYP)-resin.
[0087] In the synthesis of the above NH 2 - Add 20% piperidine / N-methylpyrrolidone solution to the protected peptide (YGGFLRKYP) resin to remove the 9-fluorenylmethoxycarbonyl group bound to the amino group, and wash with N-methylpyrrolidone and dichloromethane , at room temperature, the coupling reaction was carried out with 5 equivalents of nicotinic acid for 12 hours. After the reaction, it was washed several times with N-methylpyrrolidone and dichloromethane and dried.
[0088] At room temperature, using a mixed solution of trifluoroacetic acid: phenol: thioanisole: water: ethanedithiol (82.5: 5: 5: 5: 2.5 (v / v)) to make the dried nicotinoyl-nonapeptide -The resin was reacted for 2 to 3 hours to remove the tert-butoxycarbonyl group, and after separating the nicotine-no...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com