B/L dual acidic heteropolyacid ion hybrid and preparation method and applications thereof
A dual-acid, heteropolyacid technology, applied in the preparation of sulfonates, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of easy deliquescence of chlorides, pollution of the environment, corrosion of equipment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
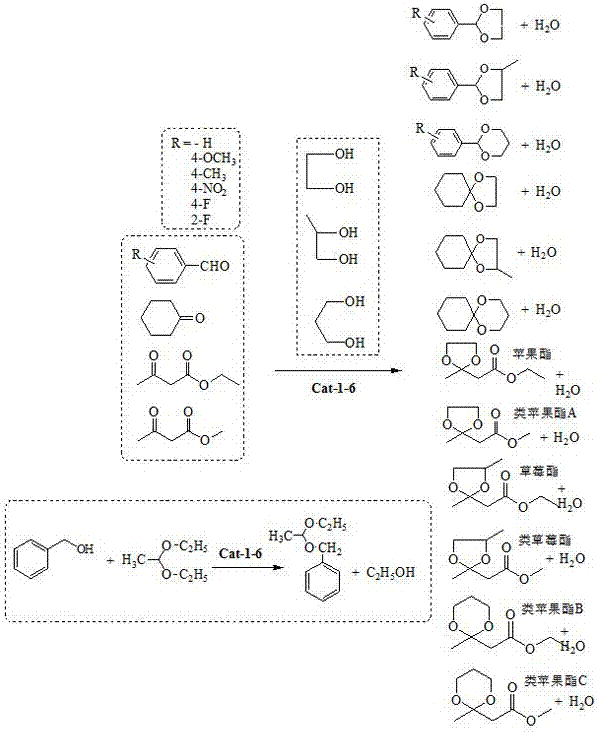
Examples
Embodiment 1
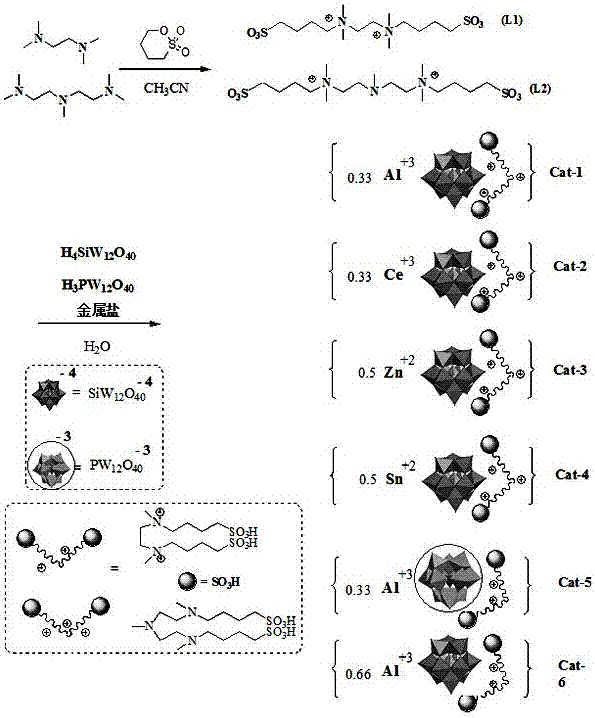
[0066] Ionic Hybrid [N 3 (SO 3 h) 2 ]Al 0.33 SiW 12 O 40 Synthesis
[0067] Step S101: In a reaction flask equipped with stirring, add 9.5 g of 1,4-butane sultone and 20 ml of acetonitrile in turn, stir at room temperature for 15 minutes, add dropwise 6.0 g of pentamethyldiethylenetriamine, and heat up after the addition The reaction was carried out at 45 °C for 24 h; the crude product was filtered with suction and rinsed with ether, and then dried under vacuum at 75 °C for 8 hours to obtain 14.5 g of the intermediate amphoteric salt L2 with a yield of 93.5%;
[0068] L2 is an off-white solid, m.p. 58°C. 1 HNMR (D 2 O, 400 MHz,), δ : 2.16~2.25(m, 8H, 2CH 2 ); 2.26(s, 3H, CH 3 ); 2.934~2.97(m, 8H, 4CH 2 ); 3.13 (s, 12H, 4CH 3 ); 3.47~3.52 (m, 8H, 4CH 2 ). 13 CNMR (D 2 O, 100 MHz), δ : 32.13 (CH 2 CH 2 N), 34.42 (CH 2 CH 2 CH 2 N), 43.35 (NCH 3 ), 48.14 (CH 2 SO 3 ), 51.85((NCH 3 ),, 57.64 (NCH 2 ), 63.67 (N + CH 2 ), 64.24 (CH 2 N + ).
[0069] ...
Embodiment 2
[0072] [N 3 (SO 3 h) 2 ]Ce 0.33 SiW 12 O 4 Synthesis
[0073] Step S101: For the preparation of the amphoteric salt L2, refer to step S101 of Example 1;
[0074] Step S102: the other operations are the same as those in Example 1, except that the aluminum nitrate is replaced by cerium nitrate, and the amount is 0.314 g. The target product 2.9 g was obtained with a yield of 93%.
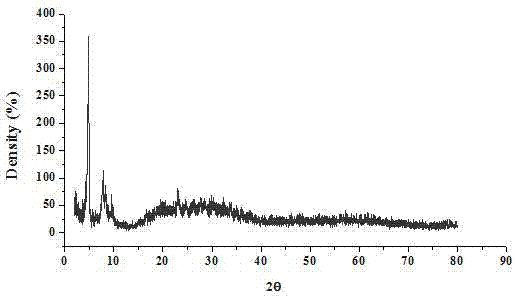
[0075] Ionic Hybrid [N 3 (SO 3 h) 2 ]Ce 0.33 SiW 12 O 4 FT-IR(KBr), ν / cm -1 : 3422 (O-H stretching vibration), 2925 (saturated-CH stretching vibration), 1040 (Si-O stretching vibration), 972 (W-O stretching vibration), 921, 793 (W-O-W stretching vibration). Elemental analysis of hybrids, C 16 H 41 N 3 SiW 12 O 46 S 2 Ce 0.33 : Found value (calculated value), %: C 5.72 (5.70), H 1.23 (1.21), N 1.25 (1.22), S 1.91, (1.88). The hybrids appear characteristic peaks of Keggin-configuration heteropolyacids in the low-angle region (5°, 8°).
Embodiment 3
[0077] Ionic Hybrid [N 3 (SO 3 h) 2 ]Zn 0.5 SiW 12 O 40 Synthesis
[0078] Step S101: For the preparation of the amphoteric salt L2, refer to step S101 of Example 1;
[0079] Step S102: The other operations are the same as in Example 1, except that zinc chloride is used instead of aluminum nitrate, and the amount is 0.149 g. The target product 2.8 g was obtained with a yield of 90%.
[0080] Ionic Hybrid [N 3 (SO 3 h) 2 ]Zn 0.5 SiW 12 O 40 FT-IR(KBr), ν / cm -1 : 3446 (O-H stretching vibration), 2925 (saturated-CH stretching vibration), 1040 (Si-O stretching vibration), 972 (W-O stretching vibration), 921, 793 (W-O-W stretching vibration). Elemental analysis of hybrids, C 16 H 41 N 3 SiW 12 O 46 S 2 Zn 0.5: measured value (calculated value), %: C 5.75 (5.71), H1.24 (1.21), N 1.26 (1.22), S 1.92, (1.90). The characteristic peaks of heteropolyacids in Keggin configuration appear in the low-angle region (5°, 8°) of the hybrid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com