Synthesis method for preparing metoprolol intermediate
A synthesis method, a technology of Metoprolol, which is applied in the directions of condensation preparation of carbonyl compounds, preparation of carbonyl compounds, chemical instruments and methods, etc., can solve the problems of high price of palladium carbon, long reaction steps, and limited industrial use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
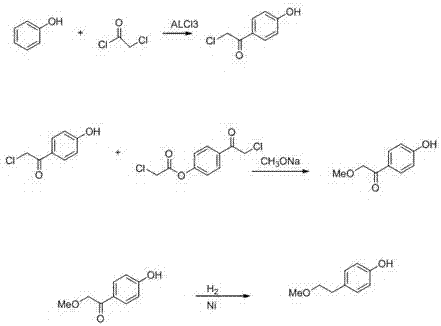
[0018] ① Dissolve 20g of phenol and 50g of chloroacetyl chloride in dichloroethane solvent, add 50g of aluminum trichloride catalyst, stir and react to generate 52.3g of 4-chloroacetylphenol;
[0019] ② Dissolve the 4-chloroacetylphenol produced by the reaction in the previous step ① in methanol, add sodium methoxide, adjust the pH to 8, turn on the stirring device to stir, and the reaction will generate 18.3g of 4-(2′-methoxyacetyl)phenol);
[0020] ③Take the 4-(2′-methoxyacetyl)phenol) produced by the reaction in step ② into the reaction kettle, add 5g of Raney nickel catalyst, feed hydrogen as a reducing agent, start stirring, heat, and control the hydrogen pressure to 0.4MPa, the reaction produced the intermediate 4-(2'-methoxyethyl)phenol.
Embodiment 2
[0022] ① Dissolve 20g of phenol and 45g of chloroacetyl chloride in dichloroethane solvent, add 60g of aluminum trichloride catalyst, stir and react to generate 48.6g of 4-chloroacetylphenol;
[0023] ② Dissolve the 4-chloroacetylphenol produced by the reaction in step ① in methanol, add sodium methoxide, adjust the pH to 9, turn on the stirring device to stir, and the reaction will generate 17.9g of 4-(2′-methoxyacetyl)phenol);
[0024] ③Take the 4-(2′-methoxyacetyl)phenol) produced by the reaction in step ② into the reaction kettle, add 6g of Raney nickel catalyst, pass in the hydrogen reducing agent, start stirring, heat, and control the hydrogen pressure to 0.45MPa , The intermediate 4-(2′-methoxyethyl)phenol was obtained by the reaction.
Embodiment 3
[0026] ① Dissolve 20g of phenol and 45g of chloroacetyl chloride in dichloroethane solvent, add 55g of aluminum trichloride catalyst, stir and react to generate 51.2g of 4-chloroacetylphenol;
[0027] ② Dissolve the 4-chloroacetylphenol produced by the reaction in step ① in methanol, add sodium methoxide, adjust the pH to 8, turn on the stirring device to stir, and the reaction will generate 20g of 4-(2′-methoxyacetyl)phenol);
[0028] ③Take the 4-(2′-methoxyacetyl)phenol produced by the reaction in the previous step ② and add it to the reaction kettle, add 5 Raney nickel catalysts, pass in the hydrogen reducing agent, start stirring, heat, and react to obtain the intermediate 4-(2'-methoxyethyl)phenol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com