Nitrogen-containing graphitized carbon material adopting double-metal MOFs (metal organic frameworks)
A graphitized carbon and bimetallic technology, applied in non-metallic elements, metal processing equipment, carbon preparation/purification, etc., can solve the problems of limited application of electrode materials, poor degree of graphitization, and low metal-nitrogen structure ratio, etc. Achieved the effect of excellent activity, excellent product performance and high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
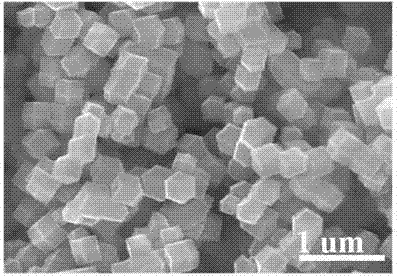
[0027] Step 1: Weigh 3.7 g of 2-methylimidazole, stir and dissolve it in 80 mL of methanol at room temperature to form solution A. Weigh 1.233 g of cobalt nitrate hexahydrate and 0.42 g of zinc nitrate hexahydrate, stir and dissolve in 80 mL of methanol at room temperature to form solution B. Slowly pour B into A, stir for 5 min, and stand at room temperature for 24 h. The mixture is centrifuged at 10,000 rpm, washed with methanol three times, vacuum-dried at low temperature for 12 h, and vacuum-activated at 200°C for 24 h. A CoZn-ZIF precursor is obtained.
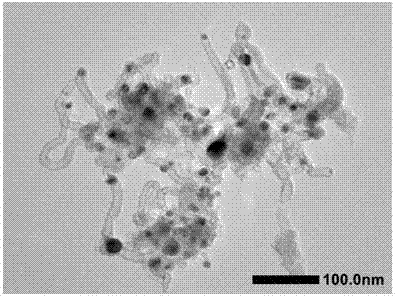
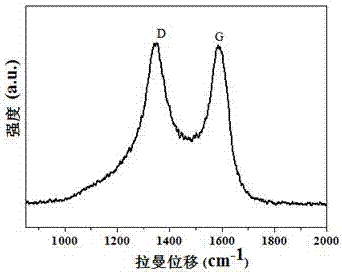
[0028] Step 2: Synthesis of Co-CNT@NC: Weigh 200 mg of CoZn-ZIF from Step 1 and put it into a tube furnace, heat it up to 900°C at a rate of 5°C / min under an argon atmosphere, and calcinate for 3 h, 35.5 mgCo-CNT@NC was obtained.
[0029] Step 3: Synthesis of P-Co-CNT@NC: Weigh 25 mg of Co-CNT@NC in Step 2 and 5 mg of triphenylphosphine in 10 mL of methanol and mix and stir for 24 h, then drain the methanol to obtain T...
Embodiment 2
[0031] Step 1: Weigh 3.7 g of 2-methylimidazole, stir and dissolve it in 80 mL of methanol at room temperature to form solution A. Weigh 0.822 g of cobalt nitrate hexahydrate and 0.84 g of zinc nitrate hexahydrate, stir and dissolve in 80 mL of methanol at room temperature to form solution B. Slowly pour B into A, stir for 5 min, and stand at room temperature for 24 h. The mixture is centrifuged at a speed of 10,000 rpm, washed with methanol for 3 times, vacuum-dried at low temperature for 12 h, and vacuum-activated at 200°C for 24 h. A CoZn-ZIF precursor is obtained.
[0032] Step 2: Synthesis of Co-CNT@NC: Weigh 200 mg of CoZn-ZIF from Step 1 and put it into a tube furnace, heat it up to 900°C at a rate of 5°C / min under an argon atmosphere, and calcinate for 3 h, 28.4 mgCo-CNT@NC was obtained.
[0033] Step 3: Synthesis of P-Co-CNT@NC: Weigh 25 mg of Co-CNT@NC in Step 2 and 5 mg of triphenylphosphine in 10 mL of methanol and mix and stir for 24 h, then drain the methanol t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com