Solid-phase PCR kit for gene detection of hypertension medication
A technology for gene detection and hypertension is applied in the field of solid-phase PCR kits for gene detection of hypertension drugs, which can solve the problems of low detection efficiency and inconvenient operation, and achieve the effects of high sensitivity, simple operation and complete detection sites.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
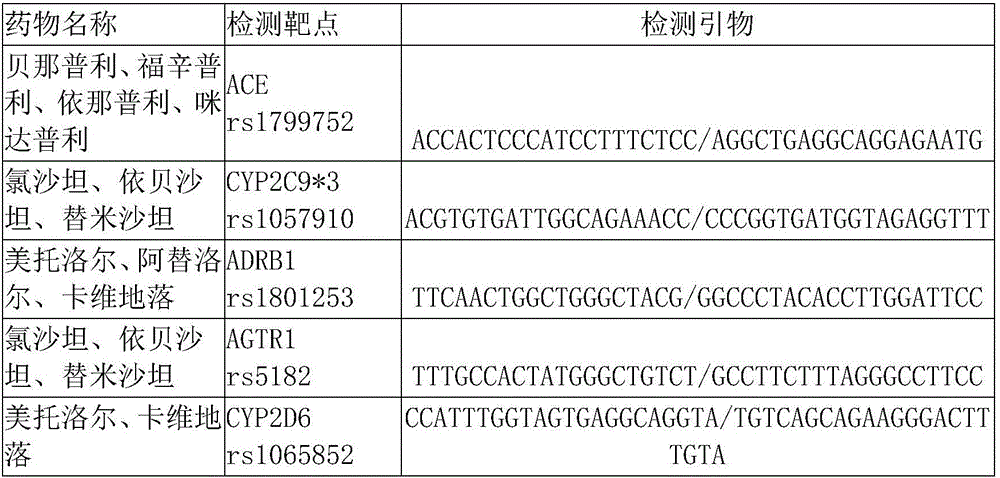
[0076] The solid-phase PCR kit for drug gene detection for hypertension consists of a PCR reaction mixture and 8-strip tubes or a 96-well PCR reaction plate, in which the PCR reaction mixture is 5×PCR Buffer (Tris-HCl pH8.5 100mM, KCl 500nM , MgCl2 15nM), dN(U)TPs(1:1:1:2), Mg2+, 5 pairs of primers and hot start DNA polymerase.
[0077] The kit is to seal a layer of paraffin wax mixture on the surface of the above PCR reaction mixture, and the paraffin wax mixture is formed by mixing solid paraffin and liquid paraffin at a ratio of 1:8 (W / V).
[0078] The reaction system (25ul) of the kit is: PCR reaction solution 23ul, template 2ul.
Embodiment 2
[0080] Kit operation and result determination
[0081] (1), extraction of genomic DNA
[0082] Use the blood / cell / tissue genome extraction kit (Tiangen Biochemical Technology, product number: DP304) to extract DNA according to the kit instructions;
[0083] (2), preparation of reaction system
[0084] Take 23ul of Hypertension Medication Solid-Phase PCR Reaction Kit 8-strip tube or 96-well PCR reaction plate, add 2ul of extracted DNA;
[0085] (3), PCR amplification
[0086] Put the PCR tube into an ordinary PCR machine for PCR amplification;
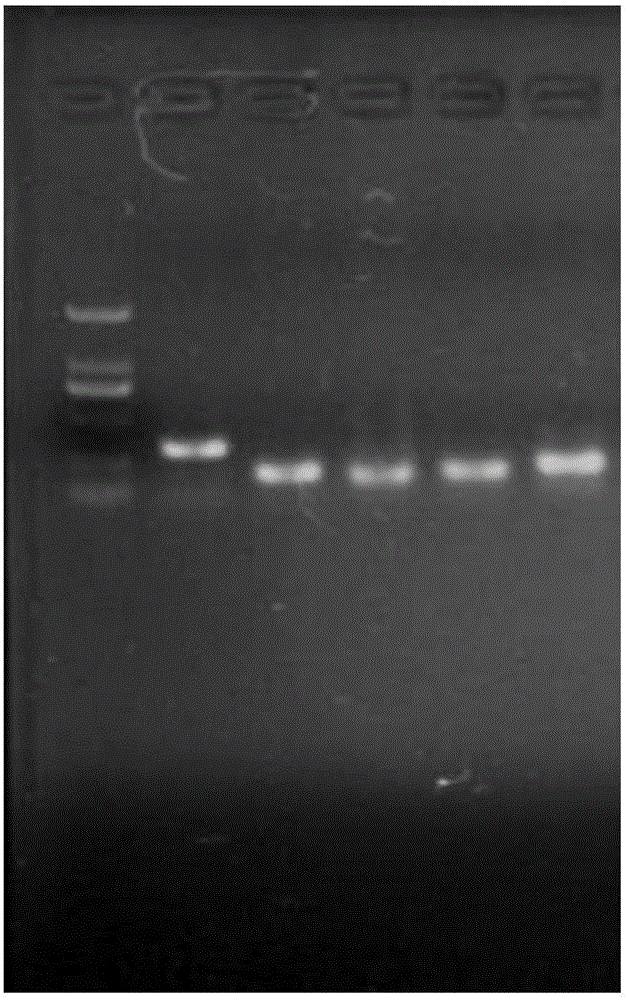
[0087] (4), 1.0% agarose gel electrophoresis analysis PCR product;
[0088] (5) Interpretation of results
[0089] The results of this kit are interpreted according to the following criteria
[0090] Table 2
[0091] detection target
Embodiment 3
[0093] Interpretation of sequencing results and medication guidance
[0094] Using the DNA extracted from one case of whole blood as a template, after amplifying with the above kit, the following sequencing results are obtained, and the corresponding medication instructions are given as follows according to the sequencing results
[0095] detection target
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com