Method for preparing highly nitrogen-doped mesoporous carbon composites
A technology of composite materials and porous carbon, which is applied in the direction of hydrocarbons, hydrocarbons, carbon compound catalysts, etc., and can solve the problems of difficult materials handling in the scale-up process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
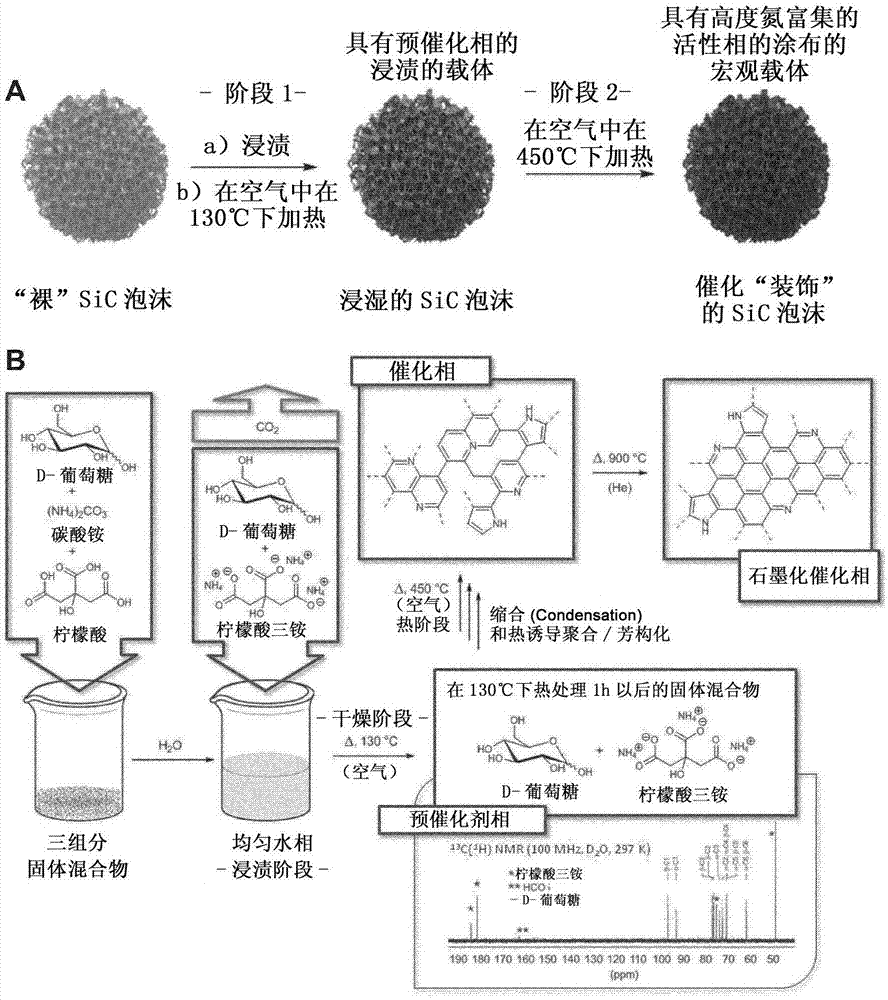
[0117] Example 1. General procedure for a highly N-rich active phase.
[0118] In a typical procedure, 2 g of dextrose and 3 g of citric acid were added to deionized water (10 mL) at room temperature (r.t.). Then, a fixed amount of ammonium carbonate (i.e., 1, 2 or 3 g) was added in a single portion to the mixture solution at room temperature and observed that due to CO 2 Instantaneous effervescence released. The suspension was stirred at room temperature until a clear solution was obtained, which was used as a source mixture for obtaining N-doped carbonaceous material deposited on a suitable support (after support soaking / impregnation). As for the latter, 2 g of different supports were used, i.e., SiC extrudates (30 m 2 g -1 ; SICAT), SiC powder (25m 2 g -1 ; SICAT), SiC foam (30m 2 g -1 ; SICAT), and α-Al 2 o 3 Beads (6m 2 g -1 ; Sasol). Slowly heat the wet solid in air: from room temperature to 130°C (10°C min -1 heating rate) and maintained at this temperature...
Embodiment 2
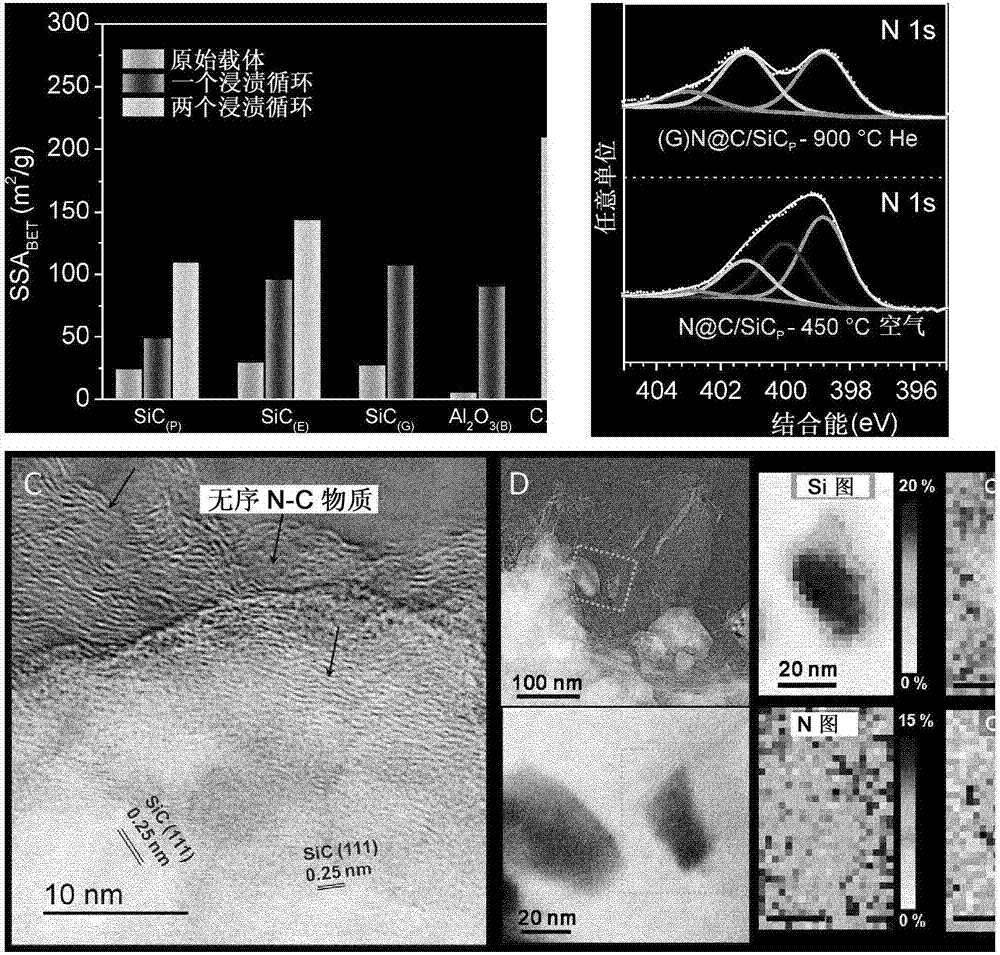
[0119] Example 2. Material Characterization.
[0120] With Bruker Avance DRX-400 spectrometer (400.13 and 100.61MHz, respectively for 1 H and 13 C) to obtain 1D ( 1 H and 13 C{ 1 H}) NMR spectrum. In ppm, relative to trimethylsilane (TMS), referenced to residual solvent resonance ( 1 H and 13 C) Chemical shifts, to report chemical shifts (δ).
[0121] With Setaram instrument, use 25mL min -1 Air flow rate and 10°C min from room temperature to 1000°C -1 The heating rate was used for thermogravimetric analysis (TGA). At liquid nitrogen temperature, by the BET method, using N 2 As an adsorbent (TriStar sorptometer), the specific surface area of the different samples was measured. Before measurement, the samples were degassed overnight at 200°C to desorb moisture and impurities on their surfaces. XPS measurements of the supports were performed with a MULTILAB 2000 (THERMO VG) spectrometer equipped with an Al Ka anode (hν = 1486.6 eV) using a 10 min acquisition. Pea...
Embodiment 3
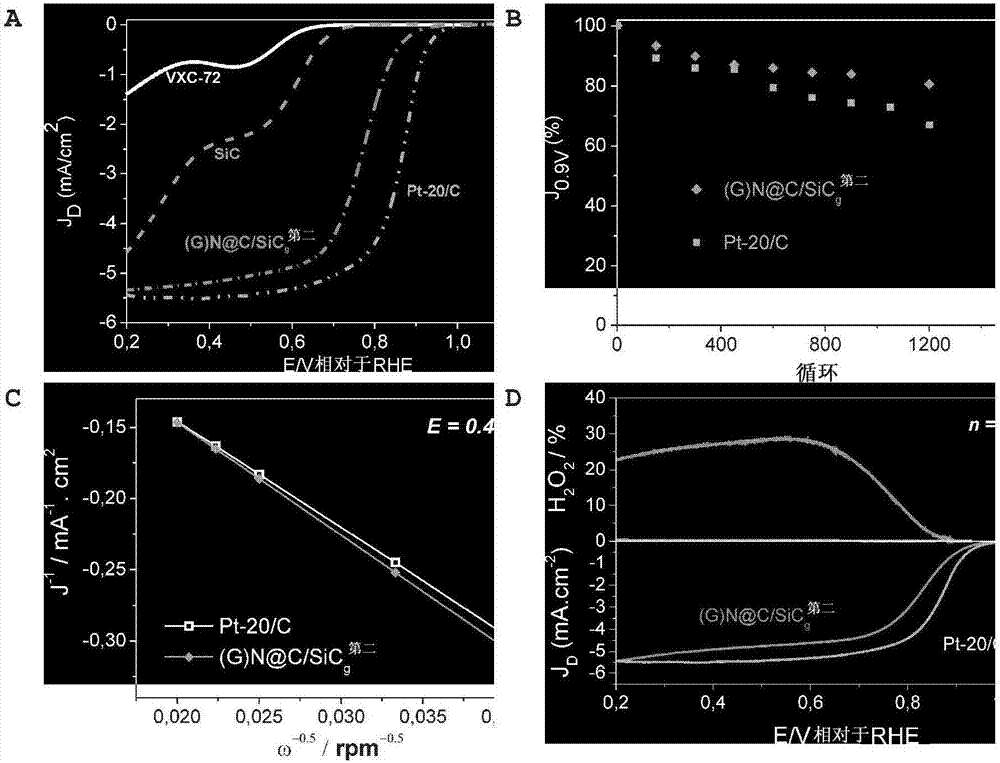
[0122] Embodiment 3. catalytic reaction
[0123] 3.1 Oxygen Reduction Reaction (ORR).
[0124] In a three-electrode cell, in 0.1 M KOH supporting electrolyte, using a device equipped with a scan rate of 10 mV s -1 Electrochemical studies were performed on an Autolab PGSTAT30 (Eco Chemie, Netherlands) potentiostat with an analog linear sweep generator at 25°C. Mercury oxide (Hg / HgO) electrode and Pt wire electrode were used as reference electrode and counter electrode, respectively. Unless otherwise stated, in the following, all potentials are referred to as reversible hydrogen electrodes (RHE). Electrochemical impedance spectroscopy (EIS) was used to determine the electrolyte solution resistance.
[0125] 10.0 mg of catalyst sample, 5 mL of isopropanol, and 50 μL of Nafion solution (5 wt %) were ultrasonically mixed to form a homogeneous catalyst ink. For defined RRDE tests, 50 μL of catalyst ink was loaded onto a preconditioned glassy carbon (GC) electrode (5.5 mm diamete...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Total measurement | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com