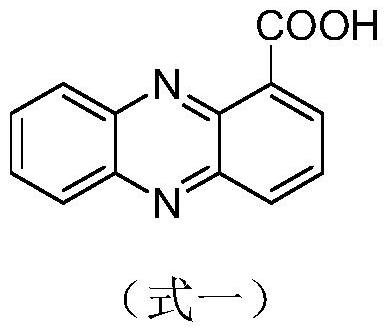
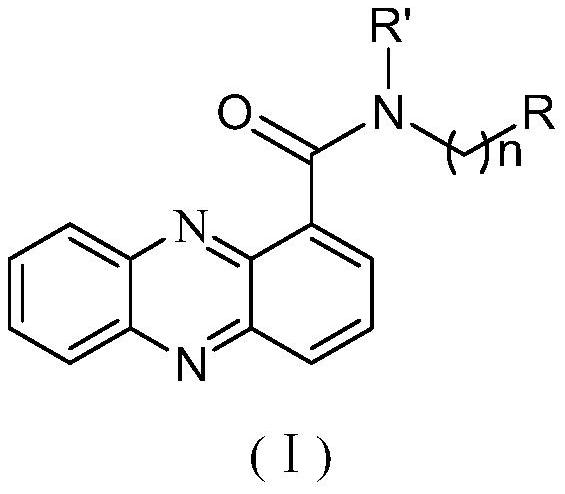
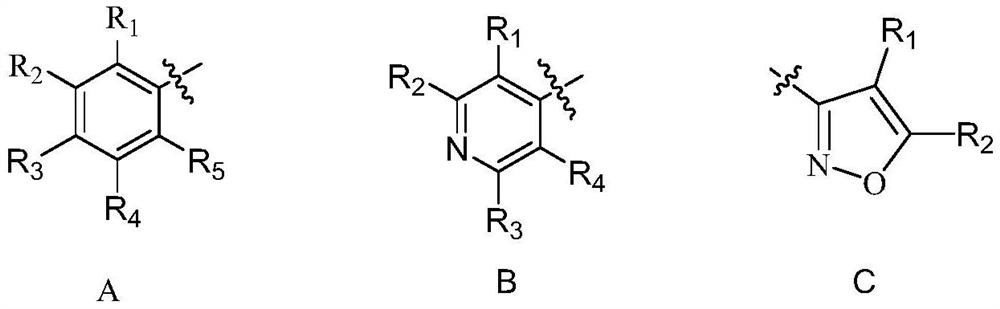
Phenazine-1-carboxamide compounds and their applications
A technology of formamides and compounds, applied in the chemical field, can solve the problems of difficult formulation processing, poor solubility of phenazine-1-carboxylic acid, etc., and achieve broad-spectrum insecticidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The synthetic method of phenazine-1-carboxamide compound of the present invention comprises the following steps:
[0052]
[0053] Below in conjunction with specific example, further set forth the present invention. It should be understood that these examples are only for illustrating the present invention and are not intended to limit the scope of the present invention. The experimental methods that do not indicate the specific implementation conditions in the following examples are usually in accordance with conventional conditions, or in accordance with the conditions suggested by the manufacturer. Percentages and parts are by mass unless otherwise indicated.
[0054] Next, taking N-(2-chloro-4-trifluoromethylphenyl)phenazine-1-carboxamide as an example, the synthesis method of the phenazine-1-carboxamide compounds of the present invention will be described.
example 1
[0055] Example 1: Synthesis of N-(2-chloro-4-trifluoromethylphenyl)phenazine-1-carboxamide:
[0056] 1) Synthesis of phenazine-1-formyl chloride:
[0057]
[0058] Add 2.5 g (11.2 mmol) of phenazine-1-carboxylic acid, 30 ml of dichloromethane into a 100 ml single-port reaction flask, drop 1 to 2 drops of DMF, slowly add 3.0 g of oxalyl chloride (to prevent flushing), and then heat to reflux Reaction until the phenazine-1-carboxylic acid completely disappears, continue to reflux for 2 to 3 hours, remove the solvent on a rotary evaporator, add a small amount of dichloromethane to dissolve, and then spin dry to take away the excess oxalyl chloride as much as possible clean, and then add a certain amount of dichloromethane to dissolve it for use in the next step.
[0059] 2) Synthesis of phenazine-1-carboxylic acid:
[0060]
[0061]Add 2.0g (10.0mmol) of 2-chloro-4-trifluoromethylaniline and 30ml of dichloromethane into a 100mL three-necked flask, cool in an ice-water bat...
example 2
[0062] Example 2: Synthesis of N-(3-chloropyridin-4-yl)phenazine-1-carboxamide:
[0063]
[0064] Add 1.3g (10.0mmol) of 3-chloro-4-aminopyridine and 30ml of dichloromethane into a 100mL three-necked flask, cool in an ice-water bath to 0-5°C, and add dropwise the phenazine-1-methanol prepared in step 1). Acyl chloride (11.2mmol) dichloromethane solution, after dropwise addition, keep warm at 0-5°C for 1 hour, monitor by pointing plate, the reaction is complete. Remove the solvent, add 50ml of dichloromethane to dissolve, fully wash the organic layer with 5% hydrochloric acid aqueous solution, separate the organic layer, then wash the organic layer with 5% aqueous sodium hydroxide solution, separate the organic layer, and dry with anhydrous sodium sulfate After 1 hour, suction filtration was performed, and the filtrate was desolvated to obtain 3.15 g of the above-mentioned amide. Yield 94%. m.p.206.2~208.1℃, 1 H NMR (600MHz, CDCl 3 )δ:13.52(s,1H),9.12(dd,J=8.4,1.8Hz,1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com