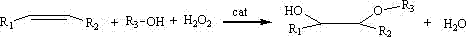Green synthesis method for preparing alcohol ether by using olefin in one step
A green synthesis and olefin technology, applied in ether preparation, organic chemistry, etc., can solve the problems of two-stage reaction, limitation, and many reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] According to the amount of 2% of TS-1 loaded copper oxide, copper hydroxide is impregnated and loaded, filtered, dried, and roasted to obtain a titanium-silicon molecular sieve copper catalyst. Other operations are the same as in Comparative Example 1. The membrane side clear liquid is analyzed. The selectivity is 99.1%. The clear liquid is continuously sent to the deene-dealcoholization tower to remove olefins and alcohols and returned to continue the reaction, and the bottom liquid is sent to the rectification tower for rectification to obtain alcohol ether products with a purity of 99.5% and a yield of 98.1%.
Embodiment 2
[0021] Carry out zinc hydroxide impregnation loading by the amount of Ti-MCM-41 zinc oxide 3%, filter, dry, roast, obtain titanium silicon molecular sieve zinc catalyst, 1-butene: ethanol: 50% hydrogen peroxide: catalyst is 15:5: 1:0.08 was tested, and the membrane side clear liquid was continuously fed into the deene dealcoholization tower to remove olefins and alcohols and returned to continue the reaction, and the bottom liquid was rectified into a rectification tower to obtain alcohol ether products with a purity of 99.6% and a yield of 98.3 %.
Embodiment 3
[0023] Carry out nickel nitrate impregnation loading according to the amount of Ti-SBA-15 loaded nickel oxide 10%, filter, dry, roast, obtain titanium silicon molecular sieve nickel catalyst, 2-butene: isopropanol: 70% hydrogen peroxide: catalyst is 26:10 : 1:0.1 is tested, and the film side clear liquid is continuously entered into the deene dealcoholization tower to remove olefins and alcohols and returns to continue the reaction, and the still bottom liquid enters the rectification tower to rectify to obtain the alcohol ether product, the purity is 99.5%, and the yield is 98.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com