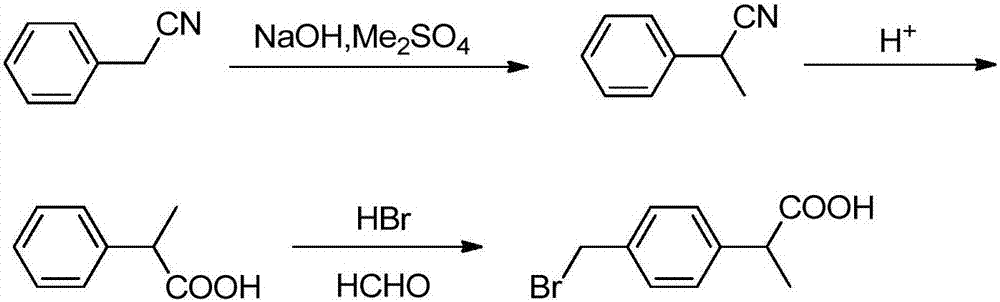
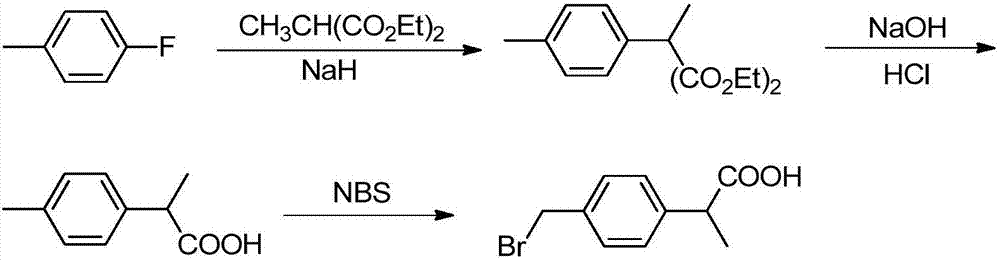
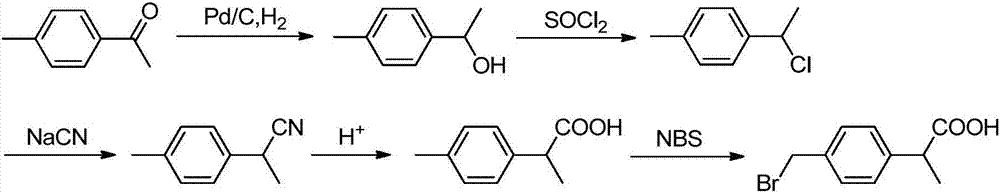
Preparation method of 2-(4-bromomethyl)phenyl propionic acid
A technology of phenylpropionic acid and bromomethyl, which is applied in the field of preparation of 2-phenylpropionic acid, can solve the problem of high cost and achieve the effects of low toxicity, high purity and simple reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050]
[0051] Put 100g of compound 1 and 435g of concentrated hydrochloric acid into the reaction flask, under nitrogen protection, start stirring, raise the temperature to 65-70°C, and keep the reaction for 6 hours. TLC detection, after the reaction is complete, cool down to 20-30°C, stand still, separate the liquid, and collect the upper organic phase. The organic phase was washed with water for 3-4 times, dried over anhydrous sodium sulfate, and filtered to remove the desiccant to obtain light yellow liquid compound 2 with a yield of 123.5 g and a yield of 94.3%. directly used in the next reaction.
[0052] 1 H NMR (500MHz, CDCl 3 ): δ7.33(d, J=7.9Hz, 2H), 7.25(d, J=7.8Hz, 2H), 5.02(m, 1H), 2.36(s, 3H), 1.76(d, J=7.1Hz ,3H). MS: 155-156 (MH). HPLC: XDB-C18 4.6*250, methanol / water 1:1, 254nm, 45min.
Embodiment 2
[0054]
[0055] Put 60g of compound 1 and 260g of concentrated hydrochloric acid into the reaction bottle, protect it with nitrogen, start stirring, raise the temperature to 65-70°C, and keep it warm for 6 hours. TLC detection, after the reaction is complete, cool down to 20-30°C, stand still, separate the liquid, and collect the upper organic phase. The organic phase was washed with water for 3-4 times, dried over anhydrous sodium sulfate, and filtered to remove the desiccant to obtain light yellow liquid compound 2 with a yield of 73.8 g and a yield of 94%. directly used in the next reaction.
[0056] 1 H NMR (500MHz, CDCl 3 ): δ7.33(d, J=7.9Hz, 2H), 7.25(d, J=7.8Hz, 2H), 5.02(m, 1H), 2.36(s, 3H), 1.76(d, J=7.1Hz ,3H). MS: 155-156 (MH). HPLC: XDB-C18 4.6*250, methanol / water 1:1, 254nm, 45min.
Embodiment 3
[0058]
[0059] Put 70g of compound 1 and 600g of hydrobromic acid (40% content) into the reaction flask, protect it with nitrogen, start stirring, raise the temperature to 60-65°C, and keep the reaction for 5 hours. TLC detection, after the reaction is complete, cool down to 20-30°C, stand still, separate the liquid, and collect the upper organic phase. The organic phase was washed 3-4 times with water, dried over anhydrous sodium sulfate, and filtered to remove the desiccant to obtain light yellow liquid compound 3 with a yield of 108.5 g and a yield of 92%. directly used in the next reaction.
[0060] 1 H NMR (500MHz, CDCl 3 ): δ7.28 (m, 2H), 7.21 (m, 2H), 5.87 (m, 1H), 2.35 (s, 3H), 1.86 (d, J=7.8Hz, 3H). MS: 200-201 (MH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com