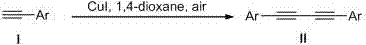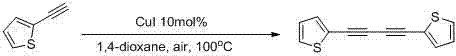Preparation method of 1,4-disubstituted-1,3-butadiyne
A diacetylene and disubstituted technology, applied in the field of organic synthesis, to achieve the effects of high yield, low production cost and easy source
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
[0028] Example 1-10 Optimization of reaction conditions
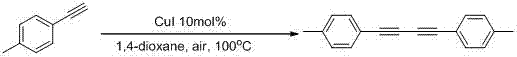
[0029] Using phenylacetylene as the raw material for the reaction, the reaction was carried out under air conditions. The effects of different catalysts, solvents and other reaction conditions on the reaction effect were explored, and representative examples 1-10 were selected. The results are shown in Table 1:
[0030]
[0031] Table I:
[0032] Example
Catalyst (mol%)
T ( o C)
Yield (%)
1
CuI(10 mol %)
DMSO
100
45
2
CuI(10 mol %)
DMF
100
37
3
CuI(10 mol %)
MeOH
100
12
4
CuI(10 mol %)
DCM
100
5
5
CuI(10 mol %)
Dioxane
100
93
6
CuI(10 mol %)
Dioxane
60
71
7
CuI(20 mol %)
Dioxane
100
95
8
CuCl(10 mol %)
Dioxane
100
63
9
CuBr(10 mol %)
Dioxane
100
72
10
CuOTf(10 mol %)
Dioxane
100
56
[0033] The basic reaction conditions are as follows: 0.4 mmol of phenylacetylene, 2 mL of solvent, and reaction time of 12 hours.
[0034] Taking Example 5 as an example, the specific operation is as follows: add 40.8 mg (0.4 mmol) ...
Embodiment 6
[0036] The difference between Example 6 and Example 5 lies in the reaction temperature. From Example 6, it can be seen that when the reaction temperature drops to 60°C, the target product 1,4-diphenyl-1,3-butadiyne is The yield dropped to 71% accordingly.
Embodiment 7
[0037] The difference between Example 7 and Example 5 lies in the amount of catalyst added. It can be seen from Example 7 that when the amount of catalyst added is 20 mol %, the target product 1,4-diphenyl-1,3- The yield of diacetylene is not significantly improved. From the perspective of production cost, the present invention selects 10 mol% of the catalyst as the optimal feeding amount.
[0038] Examples 8-10 further screened other monovalent copper catalysts, and the results showed that the catalytic effects of cuprous chloride, cuprous bromide and CuOTf on the reaction were not as good as cuprous iodide.
[0039] Taking the reaction conditions of Example 5 as the optimal conditions, the substrate expansion reaction was carried out, and a series of 1,4-disubstituted-1,3-butadiyne compounds were obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com