Preparation method of 3D drug composite stent for polyethylene glycol modified graphene oxide loaded quercetin cross-linked acellular dermal matrix
A decellularized dermis and polyethylene glycol technology, applied in medical science, prostheses, etc., can solve the problems of loss of immune activity, no obvious immunogenicity, etc., to improve water solubility, improve biological functions, and reduce inflammatory reactions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] A polyethylene glycol-modified graphene oxide-loaded quercetin cross-linked acellular dermal matrix 3D drug composite scaffold for diabetic skin wound repair method, comprising the following steps:
[0074] First, the whole skin of ICR mice was taken to make a circular acellular dermal matrix (ADM) scaffold;
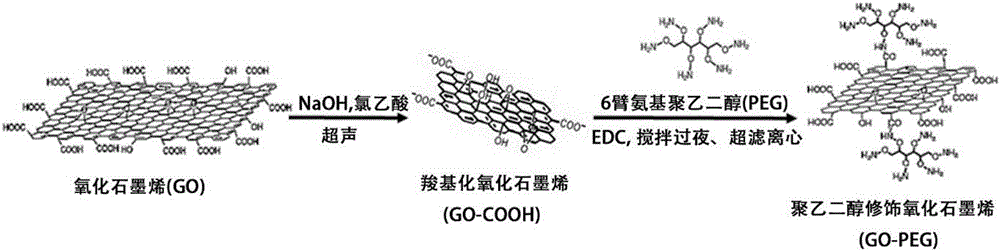
[0075] Secondly, graphene oxide (GO) was catalyzed by EDC, and 6-arm amino polyethylene glycol (PEG) was grafted onto GO to make polyethylene glycol-modified graphene oxide (GO-PEG);
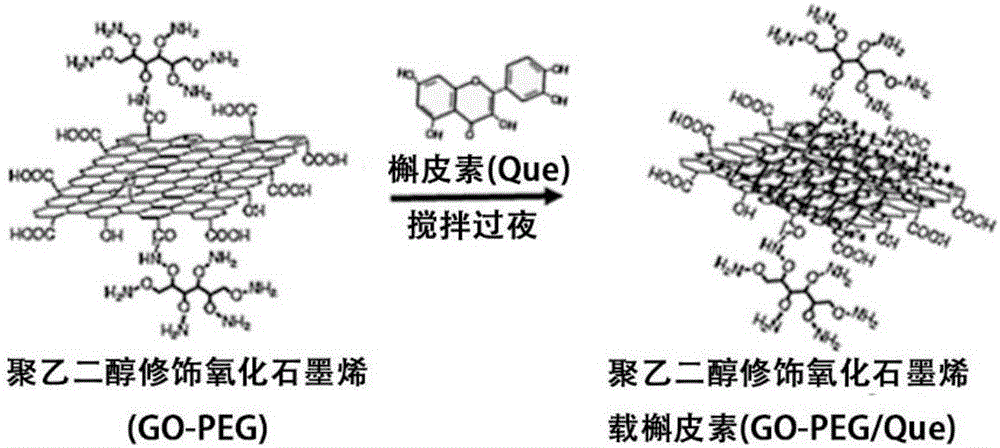
[0076] Then, adsorb quercetin (Que) onto GO-PEG, and the prepared GO-PEG / Que is cross-linked to the surface of ADM scaffold;
[0077] Finally, a 3D drug composite scaffold of polyethylene glycol-modified graphene oxide loaded with quercetin cross-linked acellular dermal matrix (ADM-GO-PEG / Que) was prepared, which can be transplanted into diabetic skin wounds to achieve Delivery of drugs and restoration of wound healing effects.
[0078] Among them, ADM is decellularized dermal mat...
Embodiment 2
[0133] (1) Production of ADM
[0134] 12-week-old male ICR mice were purchased from Guangdong Medical Experimental Animal Center. Neutral protease was purchased from Beijing Aoboxing Biotechnology Co., Ltd. (Beijing, China), and 0.25 g of powder was weighed and dissolved in 100 mL of PBS to prepare a 0.25% neutral protease solution. Both Triton X-100 (Triton X-100) and ethylenediaminetetraacetic acid (EDTA) were purchased from Tianjin Fuchen Chemical Reagent Factory (Tianjin, China), and 28.2mL Triton X-100 was mixed with 72.8mL PBS. Water bath at 37-40°C for 2-3 hours, fully dissolve and mix to make 30% Triton X-100 solution, then take 1mL 30% Triton X-100 and add it to 99mL PBS solution to make 0.3% Triton X-100 solution; Weigh 0.02g EDTA solid powder and dissolve it in 100mL PBS solution to prepare a 0.02% EDTA solution. Absolute ethanol and chloroform were purchased from Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China). Hair removal cream (Veting) was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Film diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com