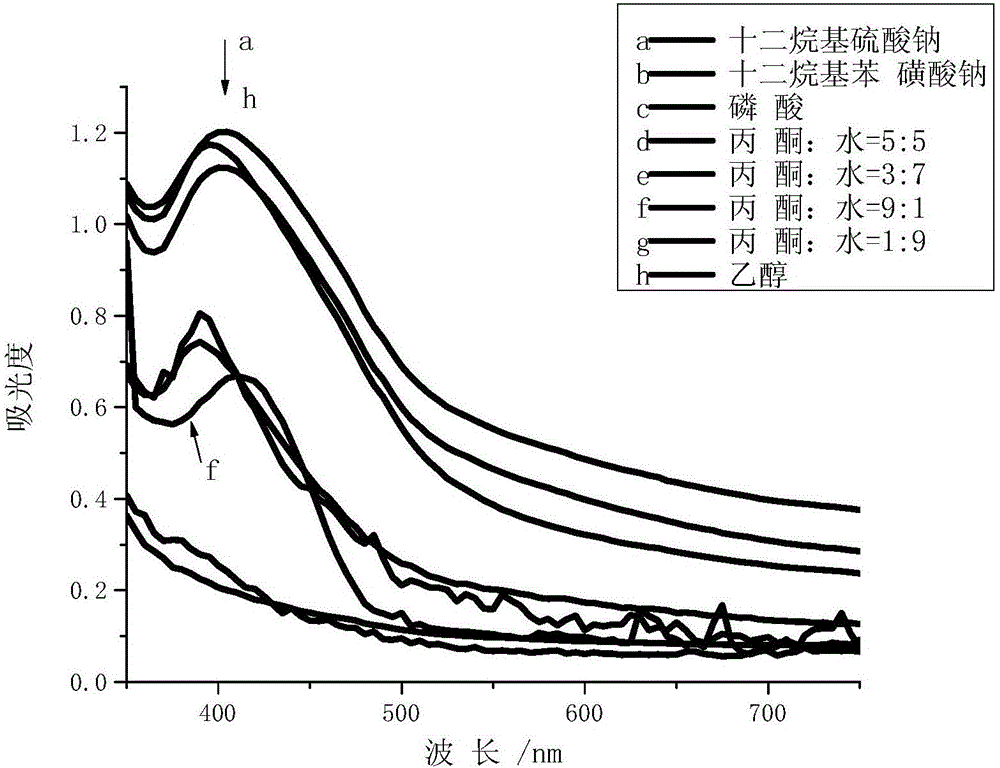
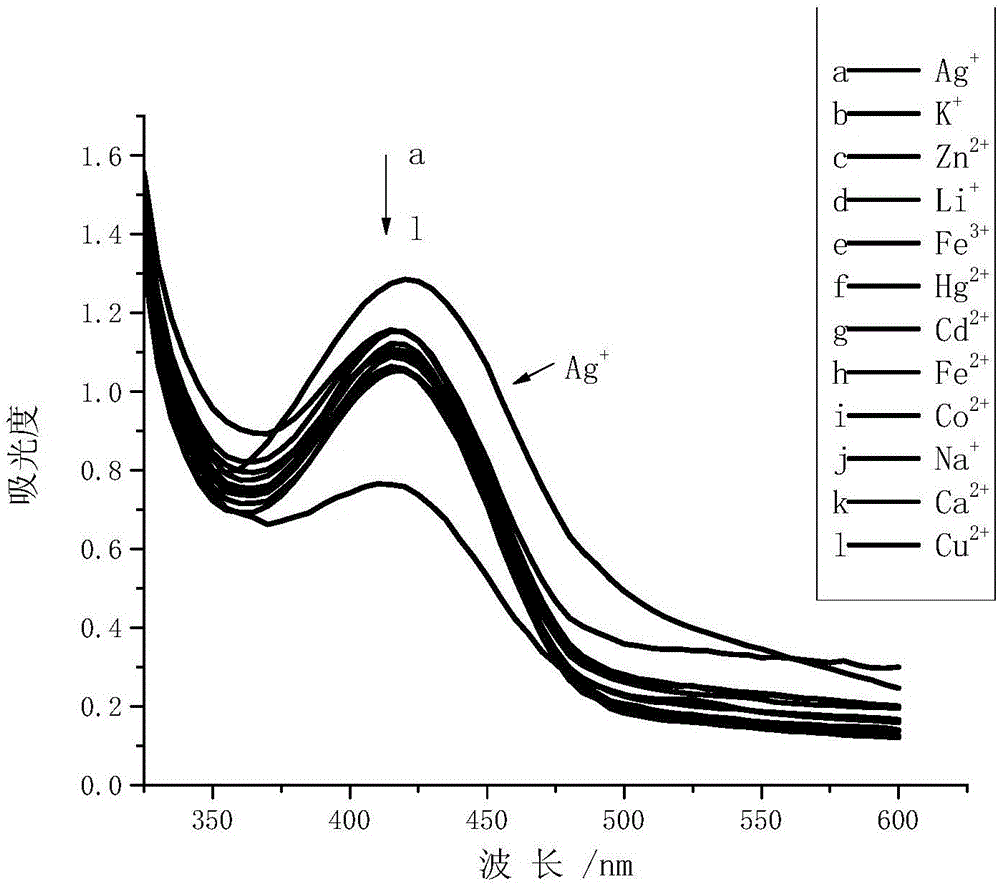
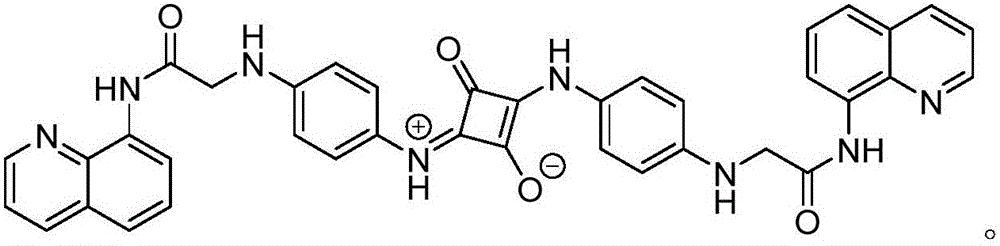
1,3-position symmetric squarylium cyanine probe based on carboxamidoquinoline as well as preparation method and application of probe
A technology based on amides and quinolines, applied in squarylium dyes, chemical instruments and methods, and analysis through chemical reactions of materials, can solve the problems of expensive analysis and achieve good selectivity, novel structure, The effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Dissolve 2-chloroacetyl chloride (0.102g, 0.9mmol) in 5mL of anhydrous CH 2 Cl 2 , then the solution was added dropwise to 30 mL of dry CH 2 Cl 2 The solution was stirred in an ice bath. After stirring at room temperature for 2 hours, the solvent was removed under reduced pressure, and by the method of column chromatography, the volume of eluent CH 2 Cl 2 :MeOH=100:1, 2-chloro-N-(quinolin-8-yl)acetamide (0.15g) was obtained as light yellow solid.
[0026] (2) Combine 2-chloro-N-(quinolin-8-yl)acetamide (0.12g, 0.545mmol) obtained in step (1) with p-phenylenediamine (0.06g, 0.55mmol), N,N -Diisopropylethylamine (DIPEA) 0.5mL, and potassium iodide (0.1g, 0.55mmol) were added to 10mL methanol solution, stirred and refluxed under nitrogen atmosphere, the mixture was cooled to room temperature, the solvent was removed under reduced pressure, and the analysis method, the volume of eluent CH 2 Cl 2 :MeOH=50:1, a yellow oil was obtained.
[0027] (3) Dissolve the y...
Embodiment 2
[0031] (1) Dissolve 2-chloroacetyl chloride (0.2 g, 1.8 mmol) in 5 mL of anhydrous CH 2 Cl 2 , then the solution was added dropwise to 30 mL of anhydrous CH 2 Cl 2 The solution was stirred in an ice bath. After stirring at room temperature for 2 hours, the solvent was removed under reduced pressure, and by the method of column chromatography, the volume of eluent CH 2 Cl 2 :MeOH=100:1, 2-chloro-N-(quinolin-8-yl)acetamide (0.2g) was obtained as light yellow solid.
[0032] (2) 2-chloro-N-(quinolin-8-yl)acetamide (0.15g, 0.68mmol) obtained in step (1) was mixed with p-phenylenediamine (0.075g, 0.68mmol), N,N -Diisopropylethylamine (DIPEA) 0.65mL, and potassium iodide (0.25g, 0.68mmol) were added to 10mL methanol solution, stirred and refluxed under nitrogen atmosphere, by column chromatography, the volume of eluent CH 2 Cl 2:MeOH=50:1, a yellow oil was obtained.
[0033] (3) Dissolve the yellow oil (0.15g, 0.49mmol) and squarylium (0.0285g, 0.25mmol) obtained in step (2)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com