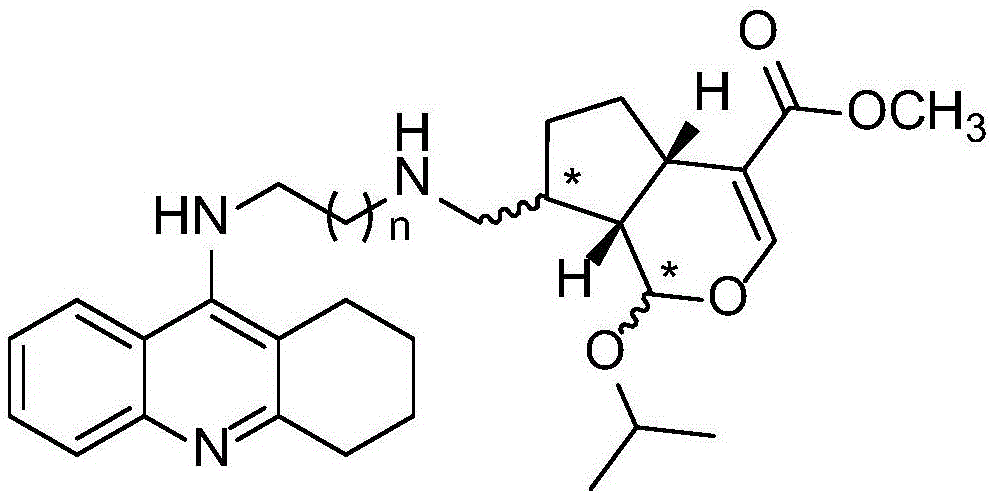
CHR20/21-tacrine heterozygote compound as well as preparation method and application thereof
A CHR20, compound technology, applied in organic chemistry, drug combination, nervous system diseases, etc., can solve problems such as lack of therapeutic drugs and in-depth research.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1: 10-N-(2-(10-amino CHR20 / 21) amino) ethyl tacrine (TNC-1)
[0087] Synthesis includes the following steps:
[0088] (1) Preparation of anthranilic acid: take methyl anthranilic acid (12.1g, 80mmol) in a 250mL round-bottomed flask, add 50mL of saturated aqueous sodium hydroxide solution, and heat in an oil bath at 105°C for 2.5 after h. After the reaction is completed, cool to room temperature. At this time, the solution is orange-yellow. Use 1.0mol / L dilute hydrochloric acid to adjust the pH value of the system to about 3-4. At this time, a small amount of white solid precipitates out; add glacial acetic acid dropwise to the reaction system, and a large amount of A white solid was precipitated, filtered with suction, the filter cake was washed with water, and dried in vacuo to obtain 10.4 g of a white solid with a yield of 95%. Melting point 144-146°C (literature value: 144-146°C) (Carlier P.R., Han Y.F., Chow E.S. Bioorg. Med. Chem. 1999, 7, 351-357). The ...
Embodiment 2
[0096] Example 2: 10-N-(3-(10-amino CHR20 / 21) amino) n-propyl tacrine (TNC-2)
[0097] Synthesis includes the following steps:
[0098] (1) Preparation of intermediate compound T-4: prepared according to steps (1) to (3) of Example 1;
[0099] (2) Preparation of intermediate compound 1a-dmp: prepared according to steps (5) to (6) of Example 1;
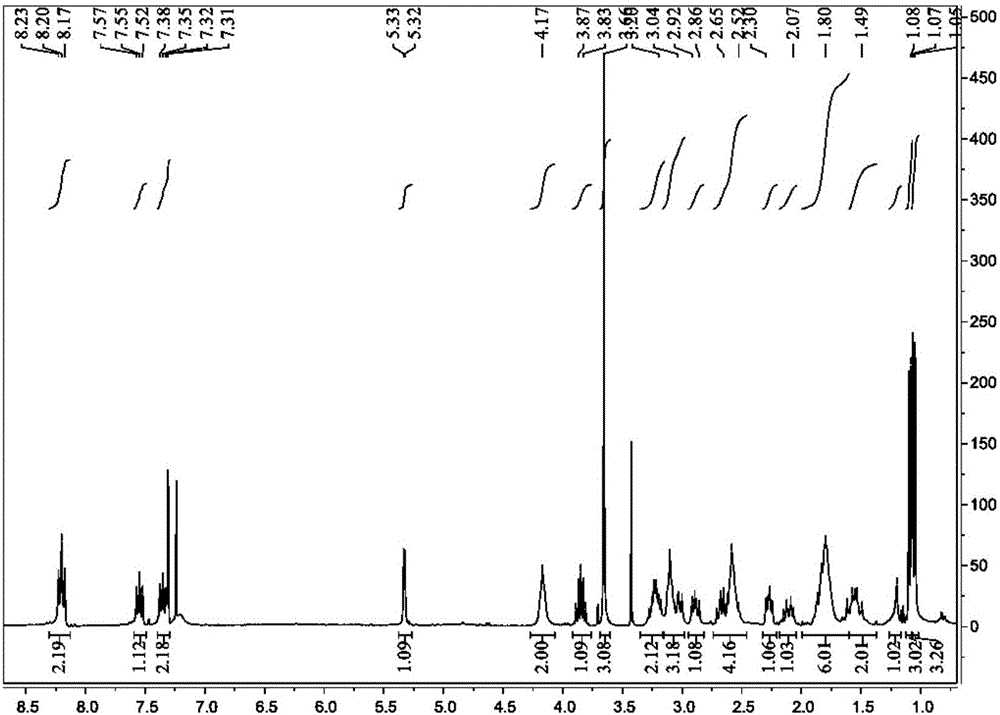
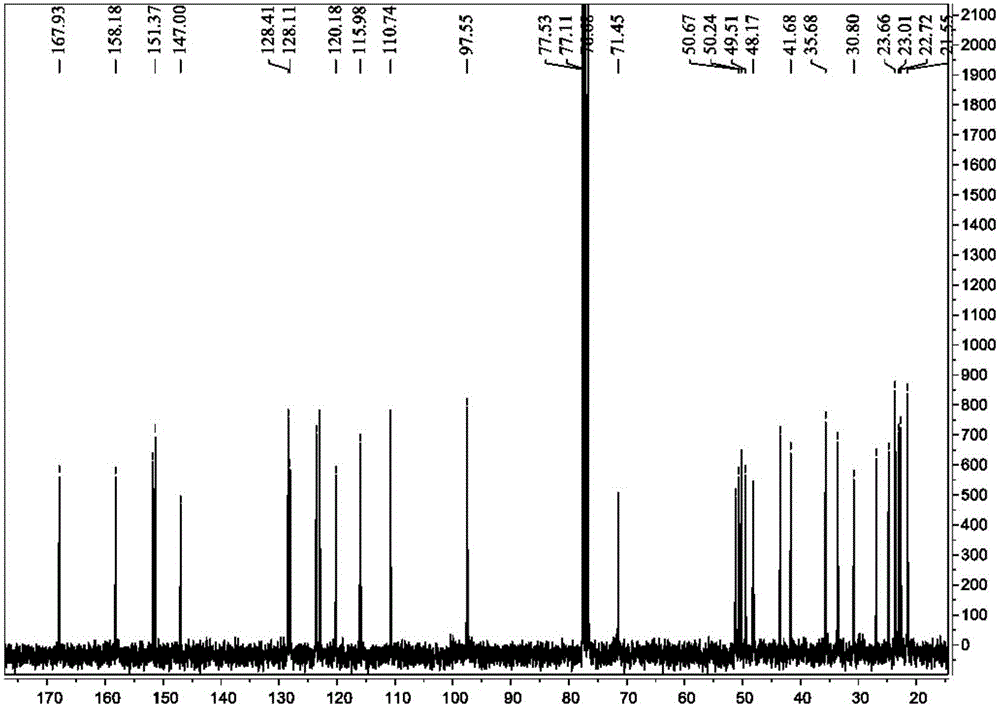
[0100] (3) Preparation of intermediate compound TA-2: prepared according to step (4) of Example 1. Ethylenediamine (8.65mmol) was replaced by 1,3-propanediamine (8.65mmol). 0.25 g of yellow oily liquid was obtained, and the yield was about 85%. 1 H NMR (300MHz, CDCl 3 )δ: 8.16-8.13(d, J=9Hz, 1H), 7.88-7.85(d, J=9Hz, 1H), 7.64-7.59(m, 1H), 7.44-7.38(m, 1H), 4.1(s , 2H, -NH2), 2.66 (t, J = 12Hz, 2H), 2.39 (t, J = 12Hz, 2H), 2.27-2.19 (m, 2H), 1.55-1.42 (m, 4H). The above data confirm that this compound is intermediate compound TA-2;
[0101] (4) Preparation of 10-N-(3-(10-amino CHR20 / 21) amino) n-propyl tacrine (TNC-2): prepared a...
Embodiment 3
[0103] Example 3: 10-N-(4-(10-amino CHR20 / 21) amino) n-butyl tacrine (TNC-3)
[0104] Synthesis includes the following steps:
[0105] (1) Preparation of intermediate compound T-4: prepared according to steps (1) to (3) of Example 1;
[0106] (2) Preparation of intermediate compound 1a-dmp: prepared according to steps (5) to (6) of Example 1;
[0107] (3) Preparation of intermediate compound TA-3: prepared according to step (4) of Example 1. Ethylenediamine (8.65mmol) was replaced by 1,4-butanediamine (8.65mmol). 0.25 g of yellow oily liquid was obtained, and the yield was about 81%. 1 H NMR (300MHz, CDCl 3 )δ:7.94-7.92(d,J=6.0Hz,1H),7.88-7.86(d,J=6.0Hz,1H),7.53-7.49(m,1H),7.32-7.29(m,1H),4.18 (s,1H,-NH-),3.47(t,J=12.0Hz,2H),3.01(m,2H),2.94(m,4H),2.71-2.66(m,4H),1.67-.62( m,2H), 1.54-1.49(m,2H). The above data confirmed that this compound was intermediate compound TA-3.
[0108] (4) Preparation of 10-N-(4-(10-amino CHR20 / 21) amino) n-butyl tacrine (TNC-3): prepared acc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com