A kind of bionic cartilage based on 3D printing and its manufacturing method
A 3D printing and cartilage technology, applied in the field of biomedical engineering, can solve problems such as difficult to prepare, achieve the effect of promoting healing, overcoming poor repair effect, good mechanical properties and vascularization ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] (1) Preparation of printing materials:
[0037] ① Preparation of modified type II collagen: weigh a certain amount of type II collagen (molecular weight: 1×10 6 kDa) was added to PBS phosphate at 50°C to prepare a solution with a mass concentration of 10%, and then methacrylic anhydride was added according to the volume ratio of type VII collagen PBS phosphate solution: V methacrylic anhydride = 10:0.75, in Stir the reaction at 50°C for 4 hours. After the reaction is over, place the solution obtained from the reaction in a dialysis bag with a molecular weight cut-off of 10,000 for dialysis for 7 days, and then place the obtained dialysate in a freeze dryer at -80°C for 48 hours. , the modified type II collagen was obtained, stored at room temperature, and set aside;
[0038] ② Preparation of modified hyaluronic acid: weigh a certain amount of hyaluronic acid (molecular weight 1.2~1.5×10 6 kDa) was dissolved in distilled water to prepare a hyaluronic acid solution with...
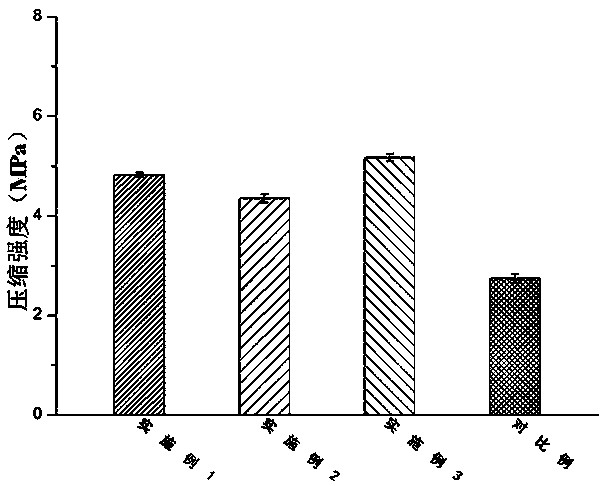
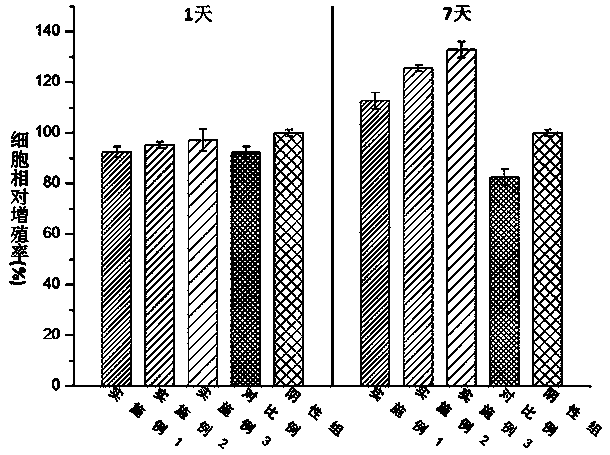
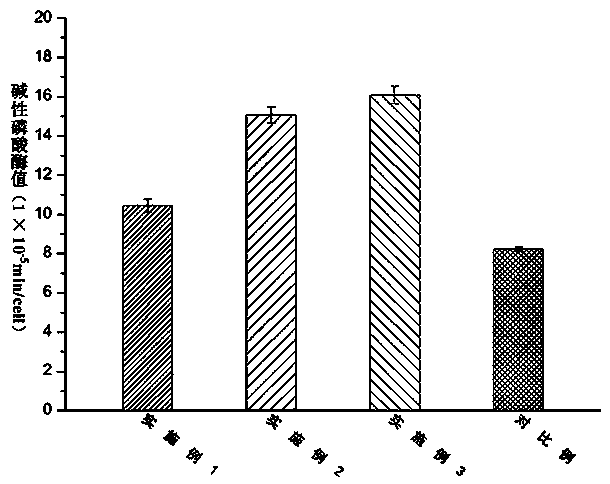
Embodiment 1
[0050] According to the present invention, a bionic cartilage based on 3D printing is prepared according to the above method, wherein: the molecular weight of type II collagen used in the preparation of the printing material is 1×10 6 kDa, the molecular weight of the hyaluronic acid used is 1.2×10 6 kDa; the particle size distribution range of nano-hydroxyapatite used in the preparation of each biomimetic layer precursor is 150-250 nm; the amount of nano-hydroxyapatite used in the preparation step of biomimetic subchondral bone precursor is 5 parts by mass, the amount of modified type II collagen is 5 parts by mass, the amount of photoinitiator Irgacure2959 is 0.5 parts by mass; the amount of modified hyaluronic acid in the preparation step of the biomimetic calcification layer precursor is 30 parts by mass, The amount of apatite used is 5 parts by mass, and the amount of photoinitiator Irgacure2959 is 0.5 parts by mass; the amount of modified hyaluronic acid used in the prepa...
Embodiment 2
[0052] According to the present invention, a bionic cartilage based on 3D printing is prepared according to the above method, wherein: the molecular weight of type II collagen used in the preparation of the printing material is 1×10 6 kDa, the molecular weight of the hyaluronic acid used is 1.2×10 6 kDa; the particle size distribution of nano-hydroxyapatite used in the preparation of each biomimetic layer precursor is 150-250 nm; the amount of nano-hydroxyapatite in the preparation step of biomimetic subchondral bone precursor is 10 mass parts, the amount of modified type II collagen is 10 parts by mass, the amount of photoinitiator Irgacure2959 is 0.5 parts by mass; The amount of limestone used is 5 parts by mass, the amount of photoinitiator Irgacure2959 is 0.5 parts by mass; the amount of modified hyaluronic acid used in the preparation step of biomimetic cartilage deep layer precursor is 15 parts by mass, and the amount of modified type II collagen is 10 parts by mass, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com