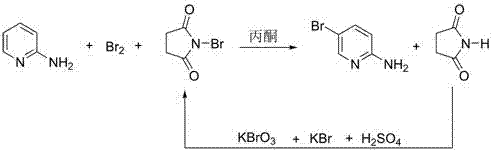
Preparation method of 2-amino-5-bromopyridine
A technology of aminopyridine and bromopyridine, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of increased reaction temperature, energy consumption, and low product yield, and achieves the goals of improving yield and purity, increasing yield, and increasing conversion rate of raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Dissolve 50 g of 2-aminopyridine in 200 mL of acetone, cool the solution to -5~-3 °C, add bromine in acetone solution (42.4 g of bromine / 200 mL of acetone) dropwise under stirring, and control the reaction temperature at - 5~0 ℃, after the dropwise addition, react for 0.5 h until the raw materials are completely reacted; the temperature of the solution is adjusted to -1~1 ℃, and 47.3 g of solid N-bromosuccinimide is added in batches, and the temperature of the reaction solution is controlled at 3~5 ℃, the reaction was continued for 1 h after the addition, and then the acetone was recovered by distillation under reduced pressure at 30 ℃ to obtain a brownish yellow solid; at 0~5℃, the brownish yellow solid was added to 94 g of potassium hydroxide aqueous solution with a mass fraction of 17.45%, and stirred , filtered, and the filter cake was recrystallized by adding 250 mL of 95% ethanol to obtain 82.7 g of brown crystals of 2-amino-5-bromopyridine, with a yield of 90% and...
Embodiment 2
[0022] Dissolve 50 g of 2-aminopyridine in 200 mL of acetone, cool the solution to -5~-3 °C, add bromine in acetone solution (42.4 g of bromine / 200 mL of acetone) dropwise under stirring, and control the reaction temperature at - 5~0 ℃, after the dropwise addition, react for 0.5 h until the raw materials are completely reacted; the temperature of the solution is adjusted to -1~1 ℃, and 47.5 g of solid N-bromosuccinimide is added in batches, and the temperature of the reaction solution is controlled at 1~3 ℃, the reaction was continued for 1 h after the feeding was completed, and then the acetone was recovered by distillation under reduced pressure at 28 ℃ to obtain a brown-yellow solid; Stir and wash, filter, and add 250 mL of 95% ethanol to the filter cake for recrystallization to obtain 82.2 g of brown crystals of 2-amino-5-bromopyridine, with a yield of 89.5% and a purity of 99.2%. The filtrate is all transferred to the next step.
[0023] Dissolve 14.8 g (89.4 mmol) of pot...
Embodiment 3
[0025] Dissolve 50 g of 2-aminopyridine in 200 mL of acetone, cool the solution to -5~-3 °C, add bromine in acetone solution (42.4 g of bromine / 200 mL of acetone) dropwise under stirring, and control the reaction temperature at - 5~0 ℃, react for 0.5 h after the dropwise addition until the raw materials are completely reacted; adjust the solution temperature to -1~1 ℃, add 49 g of solid N-bromosuccinimide in batches, and control the temperature of the reaction solution at 0~2 ℃, the reaction was continued for 1 h after the feeding was completed, and then the acetone was recovered by distillation under reduced pressure at 34 ℃ to obtain a brown-yellow solid; Wash, filter, and add 250 mL of 95% ethanol to the filter cake for recrystallization to obtain 83.6 g of brown crystals of 2-amino-5-bromopyridine, with a yield of 90.5% and a purity of 99.0%.
[0026]Dissolve 14.8 g (89.4 mmol) of potassium bromate into the succinimide aqueous solution recovered in the previous step, add 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com