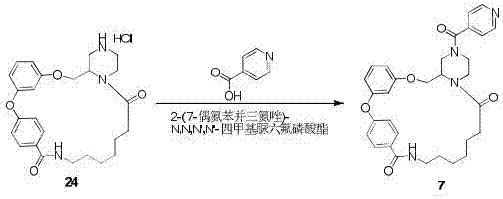
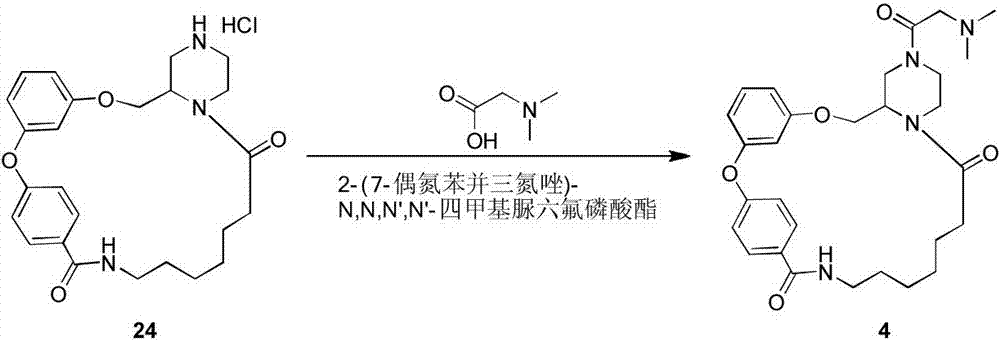
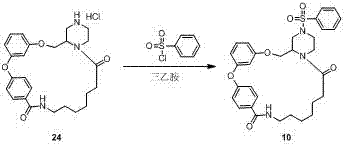
Macrocyclic lactam compound using diaryl ether as skeleton, and preparation method thereof
A technology of macrocyclic lactam and diaryl ether, which is applied in the field of macrocyclic lactam compounds, can solve the problems of difficult chemical synthesis, complex structure, and long cycle, and achieve mild process conditions, simple operation, and cheap reagents Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1) Synthesis of Compound 1
[0043] a) Synthesis of Compound 15
[0044] 10.0 g of 3-methoxyphenol (80.6 mmol), 14.8 g of ethyl 4-iodobenzoate (53.7 mmol), 3.07 g of cuprous iodide (16.1 mmol), 52.5 g of cesium carbonate (161 mmol ) and 5.54 g of 2-(dimethylamino)acetic acid (53.7 mmol) were dissolved in 120 ml of 1,4-dioxane, the reaction mixture was stirred at 80°C for 15 hours, concentrated, the organic solvent was removed, and 60 ml of water was added , extracted with dichloromethane (150 ml x 2). The organic phases were combined, dried over anhydrous sodium sulfate, spin-dried, and purified on a silica gel column (petroleum ether: ethyl acetate (volume ratio) = 1:0 to 10:1) to obtain yellow oil 15 (9.10 g, yield: 62.3 %). ESI-MS m / z : 273.0 [M+1] + .
[0045] b) Synthesis of Compound 16
[0046]4.00 g of compound 15 (15.0 mmol) was dissolved in 50 ml of dichloromethane, cooled to minus 78°C, and 4.25 ml of boron tribromide (44.1 mmol) was added. After the ...
Embodiment 2
[0066]
[0067] 2) Synthesis of Compound 2
[0068] Dissolve 35 mg of compound 24 (74 μmol) and 31 μl of triethylamine (0.22 mmol) in 5 mL of dichloromethane, and then add 8.0 μl of cyclopropylformyl chloride (89 μmol). The reaction was stirred at 20°C for 1 hour, concentrated, added with 20 mL of water, and extracted with dichloromethane (40 mL×2). The organic phase was dried with anhydrous sodium sulfate, spin-dried, and purified by high performance liquid chromatography to obtain 8.5 mg of white solid compound 2 (yield: 22.8%). ESI-MS m / z : 506.1 [M+1] + . 1 H NMR (400 MHz, DMSO- d 6 ) δ 8.45-8.33 (m, 1H), 8.04-7.93 (m, 2H), 7.28 (t, J = 8.0 Hz, 1H),7.22-7.14 (m, 2H), 6.86-6.79 (m, 1H), 6.37-6.22 (m, 1H), 4.53-4.17 (m, 3H),4.15-3.94 (m, 1H ), 3.79-3.55 (m, 2H), 3.43-3.35 (m, 1H), 3.25-2.88 (m, 3H),2.85-2.72 (m, 1H), 2.27-1.87 (m, 3H), 1.86-1.58 (m, 1H), 1.58-1.32 (m, 4H), 1.31-1.06 (m, 5H), 0.81-0.62 (m, 4H).
Embodiment 3
[0070]
[0071] 3) Synthesis of compound 3
[0072] Dissolve 35 mg of compound 24 (74 μmol) and 31 μl of triethylamine (0.22 mmol) in 5 mL of dichloromethane, and add 8.1 μl of cyclopropylformyl chloride (89 μmol). The reaction was stirred at 20°C for 1 hour, concentrated, added with 20 mL of water, and extracted with dichloromethane (40 mL×2). The organic phase was dried with anhydrous sodium sulfate, spin-dried, and purified by high-performance liquid chromatography to obtain 6.1 mg of white solid compound 3 (yield: 16.2%). ESI-MS m / z : 532.1 [M+23] + . 1 H NMR (400 MHz, DMSO- d 6) δ8.45-8.30 (m, 1H), 8.05-7.92 (m, 2H), 7.31-7.26 (m, 1H), 7.21-7.16 (m, 2H), 6.86-6.78 (m, 1H), 6.66- 6.55 (m, 1H), 6.54-6.46 (m, 0.3H), 6.27-6.24 (m, 0.7H), 4.50-4.40 (m, 0.7H), 4.16-4.30 (m, 1.3H), 4.17-4.00 (m, 3H), 3.85-3.55 (m, 3H), 3.45-3.35 (m, 1H), 3.28-3.26 (m, 4H), 3.26-3.05 (m, 3H), 2.88-2.75 (m, 1H) , 2.45-2.03 (m, 2H), 1.55-1.45 (m, 1H), 1.40-1.15 (m, 6H). 1 H NMR (400 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com