Modified block copolymer hydride with alkoxysilyl group and application thereof
A technology of block copolymers and hydrides, applied in photovoltaic power generation, electrical components, circuits, etc., can solve problems such as impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0112] Hereinafter, although an Example and a comparative example demonstrate this invention in detail, this invention is not limited only to a following example. In addition, unless otherwise specified, "part" and "%" are based on mass.
[0113] Evaluations in the present examples and comparative examples were performed by the following methods.
[0114] (1) Weight average molecular weight (Mw) and molecular weight distribution (Mw / Mn)
[0115]The molecular weights of the block copolymer (C) and the hydrogenated block copolymer (D) were measured at 38° C. as standard polystyrene-equivalent values of GPC using THF as an eluent. As a measuring device, HLC8020GPC (manufactured by TOSOH Corporation) was used.
[0116] (2) Hydrogenation rate
[0117] determination 1 The H-NMR spectrum was used to calculate the hydrogenation rate of the main chain, side chain and aromatic ring of the hydrogenated block copolymer (D).
[0118] (3) Evaluation of heat resistance
[0119] Using...
manufacture example 1
[0133] Manufacture of a sheet (G1) formed of an encapsulating material (F1) mainly composed of a modified block copolymer hydrogenated product (E1)
[0134] (Manufacture of hydrogenated block copolymer (D1))
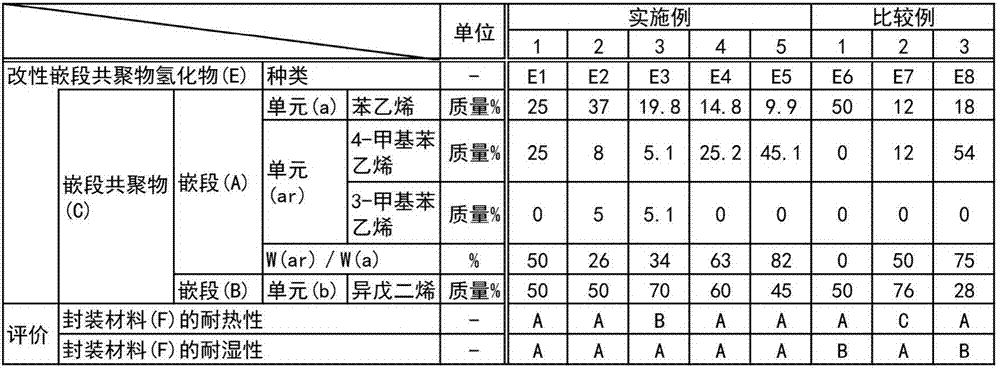
[0135] Into a tank (tank) fully nitrogen-substituted inside, put 50 parts of styrene as an aromatic vinyl compound for introducing the structural unit (a) into the block copolymer (C1), and 50 parts of 4-methylstyrene as an aromatic vinyl compound for introducing the structural unit (ar) was uniformly mixed, and passed through a dehydration tower to prepare a dehydrated aromatic vinyl compound mixture (m1).
[0136] In a reactor with a stirring device and fully carried out nitrogen replacement inside, put 400 parts of dehydrated cyclohexane, 10 parts of a mixture (m1) of aromatic vinyl compounds and 0.475 parts of dibutyl ether, while While stirring at 60°C, 0.88 parts of n-butyllithium (15% cyclohexane solution) was added to initiate polymerization. Next, while stirri...
manufacture example 2
[0153] Manufacture of a sheet (G2) formed of an encapsulating material (F2) mainly composed of a modified block copolymer hydrogenated product (E2)
[0154] (Manufacture of hydrogenated block copolymer (D2))
[0155] The mixture of the aromatic vinyl compound was changed to a mixture (m2) of 74 parts of styrene, 16 parts of 4-methylstyrene, and 10 parts of 3-methylstyrene. The same procedure as in Production Example 1 was performed except that Polymerization, hydrogenation, concentration and drying, extrusion, cooling, and granulation were carried out to produce 95 parts of granules composed of a hydrogenated block copolymer (D2).
[0156] In the block copolymer (C2), which is the precursor of the hydrogenated block copolymer (D2), wA:wB=50:50, and the structural unit (ar) accounts for all the aromatic components in the polymer block (A). The mass ratio [w(ar) / w(a)] of the total amount of structural units (a) of the vinyl compound was 26%.
[0157] The obtained hydrogenated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com