Preparation method for nefopam hydrochloride
A technique for preparing Nefopam hydrochloride and its preparation steps, which is applied in the field of preparation of Nefopam hydrochloride, and can solve problems such as complex reaction steps and difficult implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
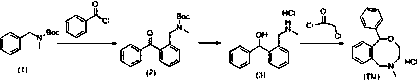
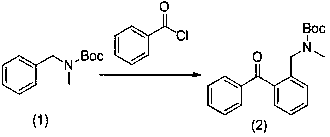
[0027] (1) For the preparation route of the intermediate N-(2-(benzoyl)benzyl)-N-methyl-carbamate tert-butyl ester, see figure 2 :
[0028] Add the raw material N-benzyl-N-methylcarbamate tert-butyl ester (22.1g, 0.1mol) and diethyl ether (100mL) into the nitrogen protection reaction system, cool down to -78°C, and slowly add n-butyllithium dropwise (44mL, 2.5M), add phenylacetyl chloride (16.7g, 0.12mol) diethyl ether solution (50mL) dropwise after 30 minutes of reaction, and react at this temperature for 1 hour after addition, then rise to room temperature, add water and stir for several minutes , separate layers, wash the organic layer with water, dry, and distill under reduced pressure to obtain 22.6 g of intermediate (2), with a yield of 70%.
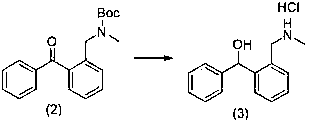
[0029] (2) For the preparation route of intermediate 1-((2-(methylamino)methyl)phenyl)-1-phenylmethanol hydrochloride see image 3 :
[0030] The intermediate N-benzyl-N-methylcarbamate tert-butyl ester (22.0g, 0.07mol), methan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com