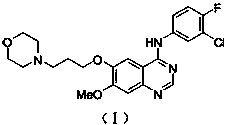
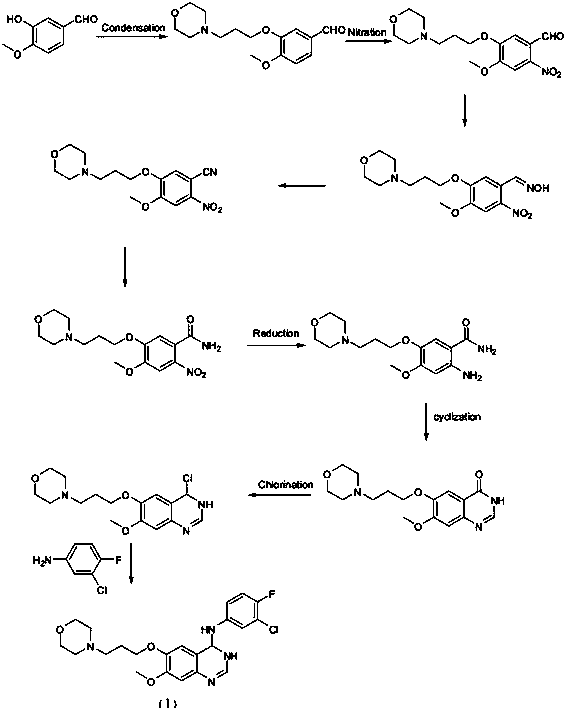
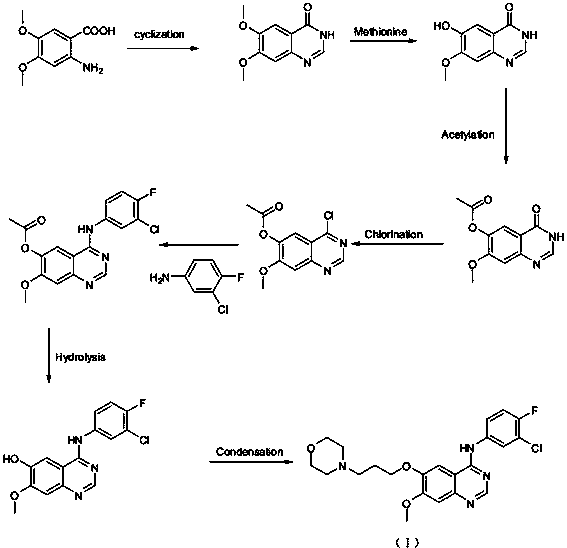
New synthesis method of gefitinib
A synthetic method, the technology of gefitinib, is applied in the new synthetic field of gefitinib, which can solve the problems of lengthy reaction steps and serious environmental pollution, and achieve the effect of less steps, simple post-processing and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Synthesis of 3-hydroxyl-4-methoxybenzonitrile (II)
[0025] 10.0g (65.8mmol) of 3-hydroxy-4-methoxybenzaldehyde (compound 1), 15mL (362mmol) of formic acid, 4.5g (65.8mmol) of sodium formate were placed in a 100mL three-necked flask, and the The temperature rose to 85°C, slowly added 22.9g (329mmol) of hydroxylamine sulfate to the reaction system within two hours, continued the reaction at this temperature for 5 hours after the addition, then stopped heating, cooled to room temperature, and poured into the reaction bottle Add 25 mL of distilled water, stir for 1 hour, filter with suction, wash with 20 mL of ice water, and dry to obtain 8.8 g (89.7%) of powdery solid.
Embodiment 2
[0026] Example 2: Synthesis of 4-methoxy-3-(3-morpholine propoxy)benzonitrile (Ⅲ)
[0027] Add 8.0g (53.6mmol) 3-hydroxy-4-methoxybenzonitrile, 10.5g (64.3mmol) 4-(3-chloropropoxy)morpholine and 56mL acetonitrile into a 100mL three-necked flask, stir, and wait for 3 After -hydroxy-4-methoxybenzonitrile is dissolved, add 14.8g (107mmol) of anhydrous potassium carbonate, heat to 85°C, react for 11 hours, cool to room temperature, remove salt by suction filtration, wash with acetonitrile, and concentrate the filtrate , precipitated a white solid, washed 3 times, weighed 14.0g after drying (yield 94.5%).
Embodiment 3
[0028] Example 3: Synthesis of 4-methoxy-5-(3-morpholine propoxy)-2-nitrobenzonitrile (Ⅳ)
[0029] Mix 13.0g (47.0mmol) of 4-methoxy-3-(3-morpholinepropoxy)benzonitrile, 17mL concentrated nitric acid (65%) (376mmol) and 17mL glacial acetic acid into a 250mL single-necked flask , Stir at room temperature for about 2 hours to precipitate a large amount of solids, continue to stir for 1 hour, stop the reaction, add 52g of ice water, stir for 30min, filter with suction, wash the filter cake with ice water to about pH 7, and dry to obtain 14.4g of white solid (95.3 %).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com