Method for producing tyrosol and hydroxytyrosol through heterologous metabolic pathways
A technology for hydroxytyrosol and tyrosol, applied in the field of heterologous metabolic pathway production of tyrosol and hydroxytyrosol, can solve the problems of long cycle, large environmental pollution, lack of production methods and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0030] Production of tyrosol and hydroxytyrosol by adding tyrosine in vitro
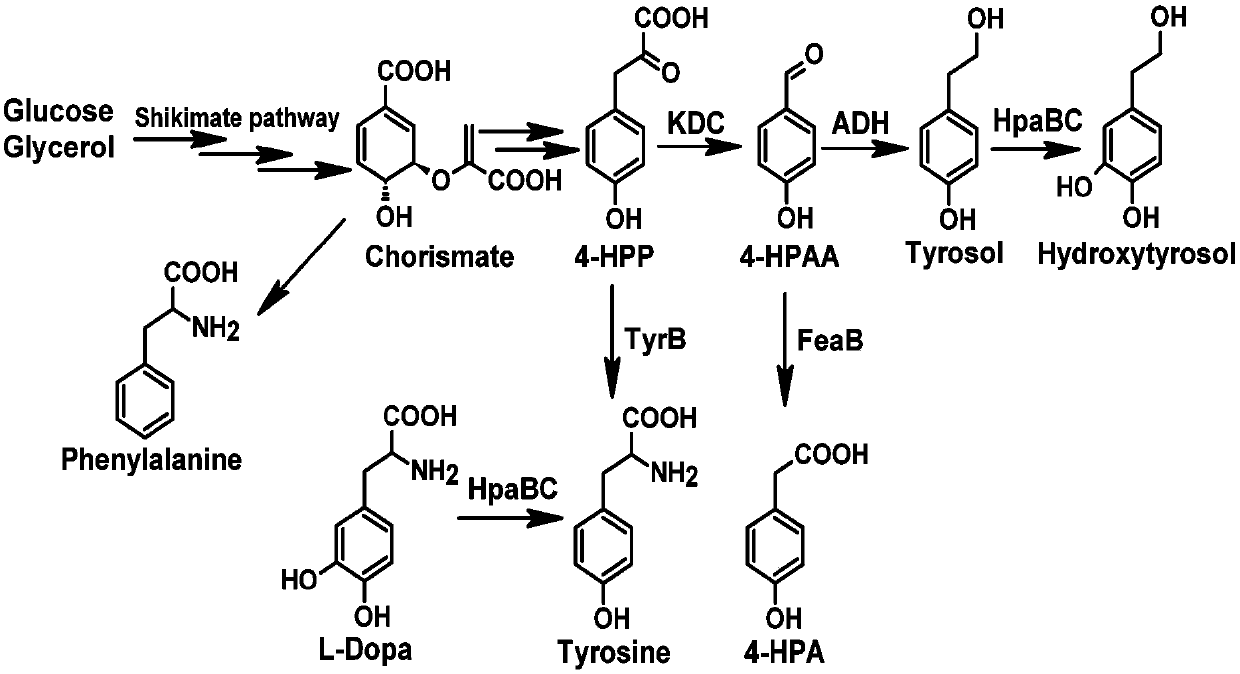
[0031] The tyrosine directly added to the medium during the fermentation process is screened from bacteria, fungi or protein engineered aminotransferase (AT), ketoacid decarboxylase (KDC), alcohol dehydrogenase (ADH) to catalyze the formation of tyrosine Alcohol, and on this basis, 4-hydroxyphenylacetic acid 3-monooxygenase (HpaBC) is introduced to catalyze the formation of hydroxytyrosol. Aminotransferase (AT), ketoacid decarboxylase (KDC), alcohol dehydrogenase (ADH), 4-hydroxyphenylacetic acid 3-monooxygenase (HpaBC) gene fragments were obtained by PCR, followed by restriction endonuclease Enzyme digestion of gene fragments and plasmid vectors, gel recovery or column recovery of the digested fragments, and then aminotransferase (AT), ketoacid decarboxylase (KDC), alcohol dehydrogenase (ADH) target gene Insert into plasmid pZE12-luc (high copy) to obtain pZE-ARO10-ADH6 recombinant plasmid; insert ...
specific Embodiment 2
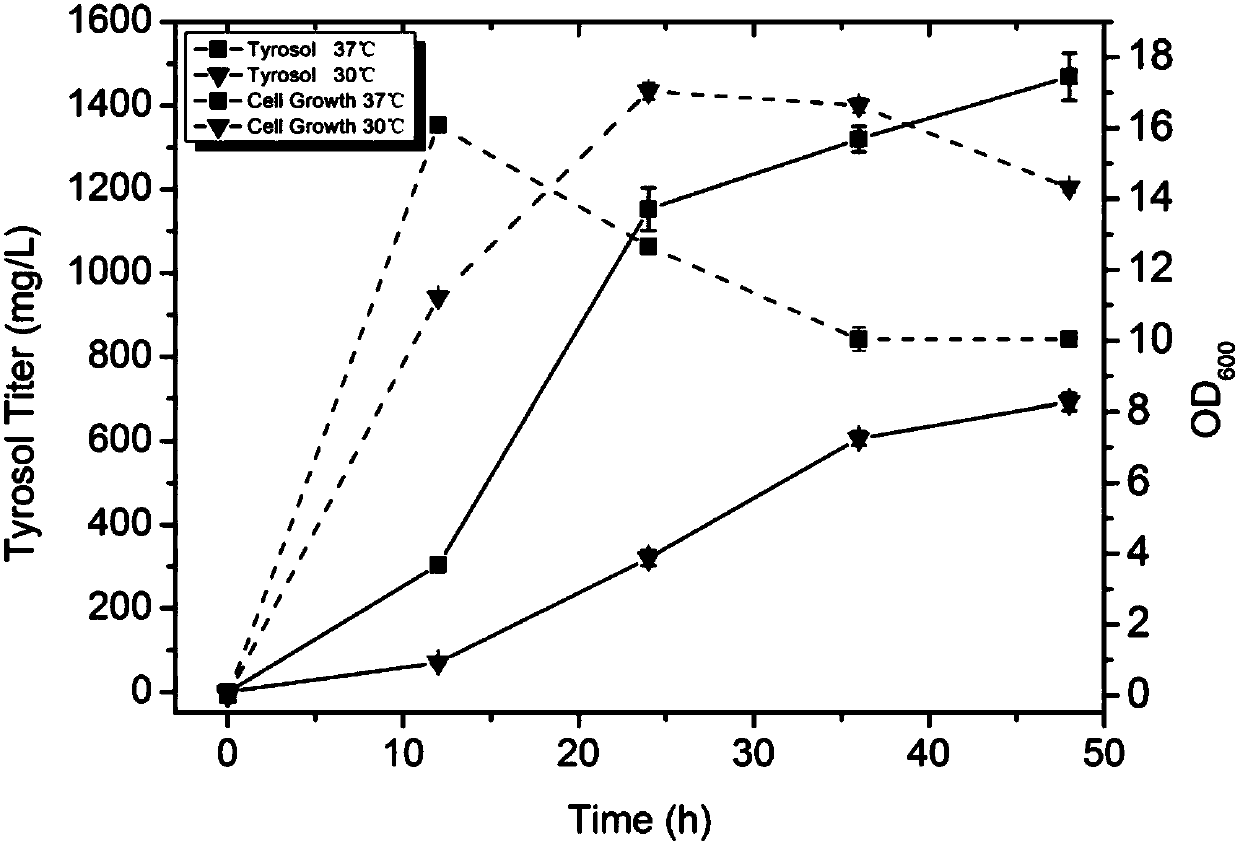
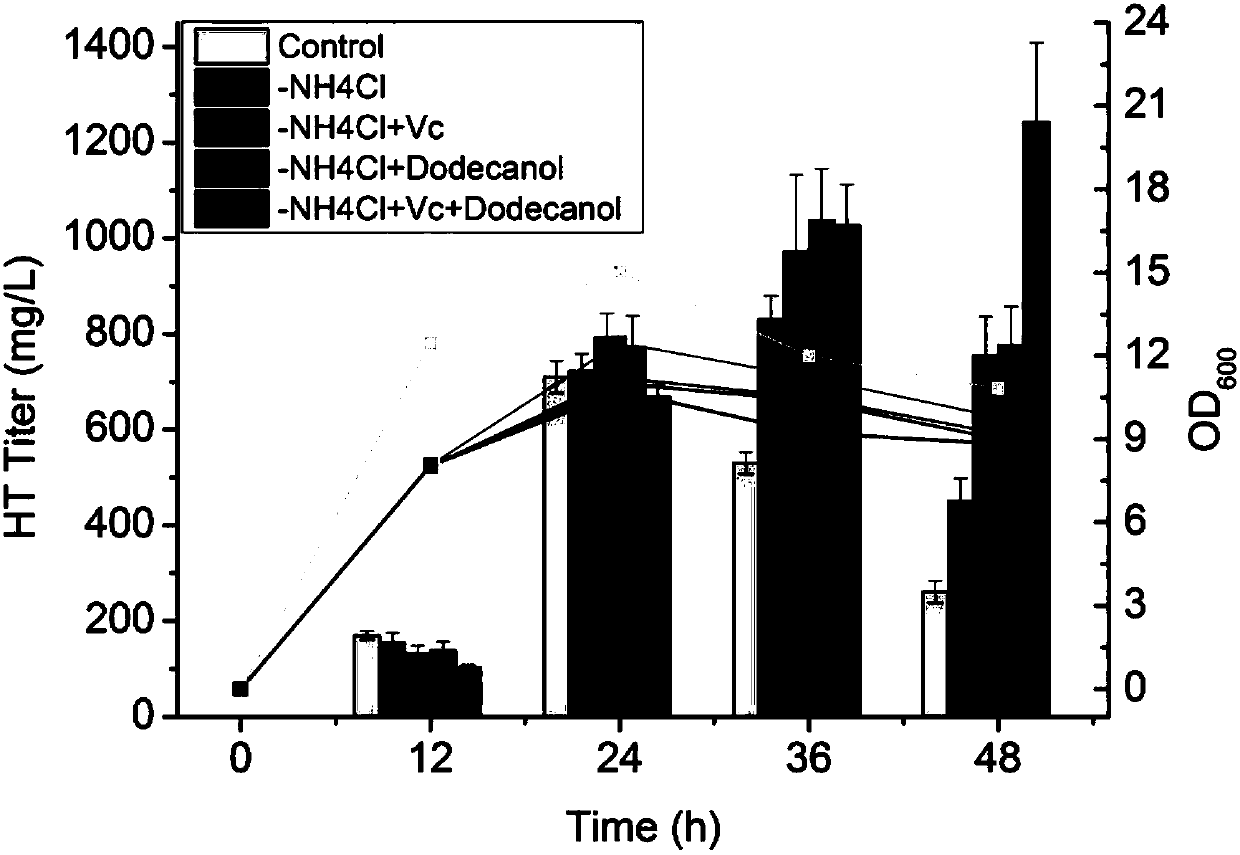
[0035] Construction of Metabolic Pathways for Microbial Heterologous Synthesis of Tyrosol and Hydroxytyrosol
[0036] Starting from glucose and glycerol, tyrosine is synthesized through the shikimate pathway, and tyrosine is screened from bacteria, fungi or protein engineered aminotransferase (AT), ketoacid decarboxylase (KDC), alcohol dehydrogenation The enzyme (ADH) catalyzes the generation of tyrosol, and on the basis of tyrosol, 4-hydroxyphenylacetic acid 3-monooxygenase (HpaBC) is introduced to catalyze the reaction of tyrosol to hydroxytyrosol. Aminotransferase (AT), ketoacid decarboxylase (KDC), alcohol dehydrogenase (ADH), 4-hydroxyphenylacetic acid 3-monooxygenase (HpaBC) and TyrA that enhances the shikimate pathway were obtained by PCR fbr , PpsA, TktA and AroG fbr Enzyme gene fragments, then use restriction endonucleases to digest the gene fragments and plasmid vectors, perform gel recovery or column recovery on the digested fragments, and then insert the target ge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com