Preparation and application of catalyst with molecular sieve as carrier for hydrogen production by methane vapor reforming
A methane water vapor, reforming hydrogen production technology, applied in the field of catalysis, can solve the problems of limited application, and achieve the effects of improving interaction, inhibiting direct decomposition reaction of methane, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
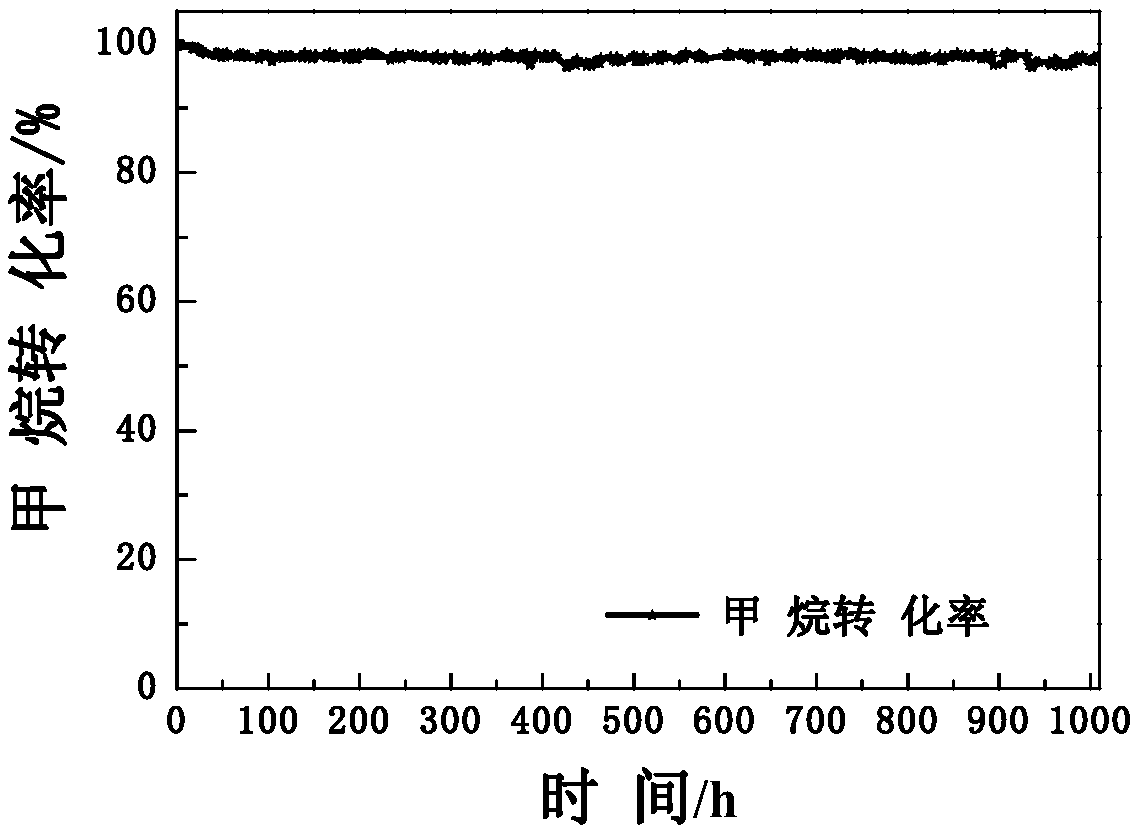
Image
Examples
Embodiment 1
[0032] Embodiment 1: Catalyst A
[0033] (1) Weigh 61.33g of water in a round-bottomed flask, then weigh 4.9029g of tetrapropylammonium hydroxide and slowly add it dropwise to the flask to form an aqueous solution, stir at 600r / min for 30min, and then rotate at 600r / min min. Slowly add 5.0895g of ethyl orthosilicate dropwise, and age for 24h.
[0034] (2) Transfer the gel system to a stainless steel reaction kettle with polytetrafluoroethylene, and perform two-stage hydrothermal crystallization. The first stage is hydrothermal crystallization at 140°C for 10 hours, and the second stage is at 175°C in an oven. Hydrothermal crystallization for 12 hours.
[0035] (3) The reaction product was centrifuged at 5000 r / min, dried at 110° C. for 12 hours, and calcined at 500° C. for 4 hours to obtain a molecular sieve carrier.
[0036] (4) This embodiment is to prepare a catalyst with a loading capacity of 10wt% NiO, that is, the mass fraction of NiO accounts for 10% of the total mas...
Embodiment 2
[0042] Example 2: Catalyst B
[0043] (1) Weigh 0.6838gZr(NO 3 ) 4 ·5H 2O and 61.33g of water were prepared into an aqueous solution, and then 4.9029g of tetrapropylammonium hydroxide was weighed and added dropwise to a round-bottomed flask, stirred at 1200r / min for 30min, after which the rotation speed was 1200r / min, and orthosilicon was slowly added dropwise Acetate ethyl 5.0895g, aged for 24h.
[0044] (2) The gel system was transferred to a stainless steel reactor with polytetrafluoroethylene, hydrothermally crystallized at 140°C for 10 hours, and then placed in an oven for hydrothermally crystallized at 175°C overnight.
[0045] (3) The reaction product was centrifuged at 5000 r / min, dried at 110° C. for 12 hours, and calcined at 500° C. for 4 hours to obtain a molecular sieve carrier.
[0046] (4) Weigh 0.4368g Ni(NO 3 ) 2 ·6H 2 O, add deionized water to dissolve, and prepare a 1.66mol / L nickel nitrate solution.
[0047] (5) Weigh 1 g of the molecular sieve carri...
Embodiment 3
[0052] Example 3: Catalyst B
[0053] (1) Weigh 0.6843gZr(NO 3 ) 4 ·5H 2 0 and 61.33g water are mixed with an aqueous solution, then take by weighing 2.5012g tetrapropylammonium hydroxide and 2.4830g tetraethylammonium hydroxide, and the two are mixed and added dropwise to 2000r / min in the round bottom flask and stirred for 30min, then The rotation speed of each was 2000r / min, and 5.0890g of ethyl orthosilicate was slowly added dropwise, and aged for 24h.
[0054] (2) The gel system was transferred to a stainless steel reactor with polytetrafluoroethylene, hydrothermally crystallized at 150°C for 12 hours, and then placed in an oven for 5h at 175°C.
[0055] (3) The reaction product was centrifuged at 5000 r / min, dried at 110° C. for 12 hours, and calcined at 500° C. for 4 hours to obtain a molecular sieve carrier.
[0056] (4) Weigh 0.4364gNi(NO 3 ) 2 ·6H 2 O, add deionized water to dissolve, and prepare a 1.66mol / L nickel nitrate solution.
[0057] (5) Weigh 1 g of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com