Sulfonating long branched chain type polyphenyl ether proton exchange membrane and preparation method thereof
A technology of proton exchange membrane and polyphenylene ether, which is used in the preparation of proton exchange membrane and the field of electrochemistry, can solve the problems of high price, limited commercial application and high vanadium ion permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
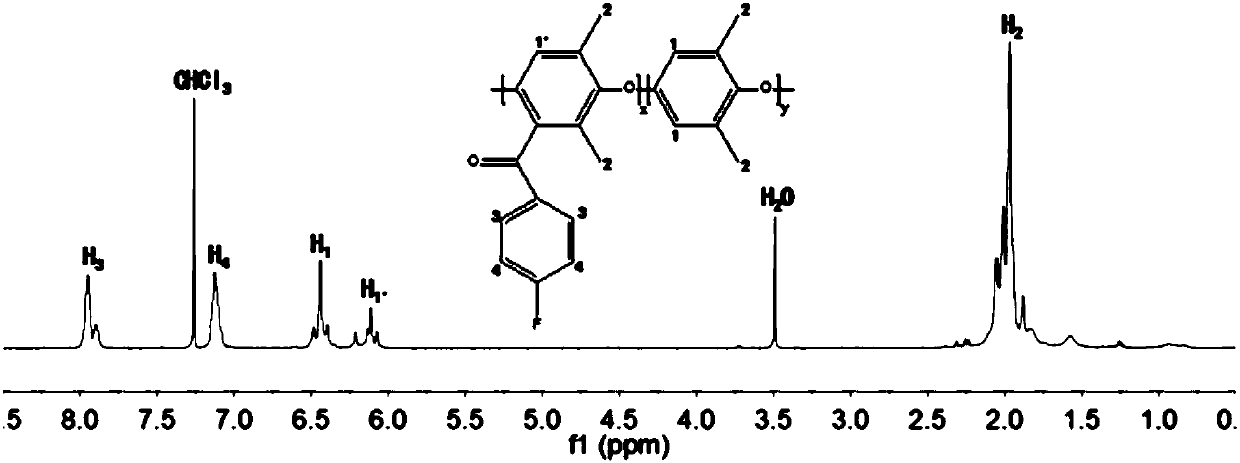
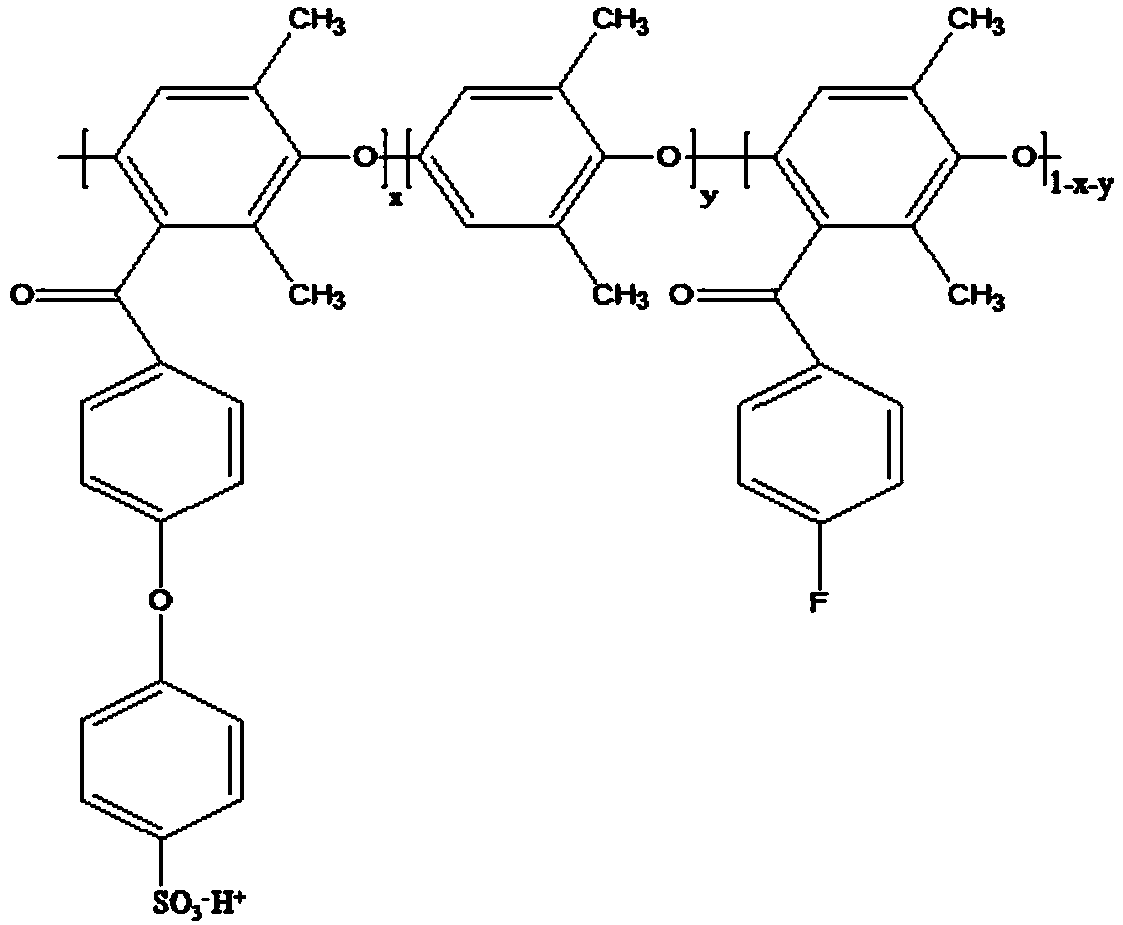
[0031] (1) Acylation of polyphenylene ether: Prepare a 1,2-dichloroethane solution of polyphenylene ether with a w / v concentration of 0.06%, add 0.8 times of polyphenylene ether under the protection of an ice-water bath and an inert gas aluminum trichloride in the amount of substance, and then add p-fluorobenzoyl chloride 0.7 times the amount of polyphenylene ether; move it to an oil bath, and react with magnetic stirring at 60°C for 10h; pour it into ethanol to precipitate, and wash repeatedly. and dry;
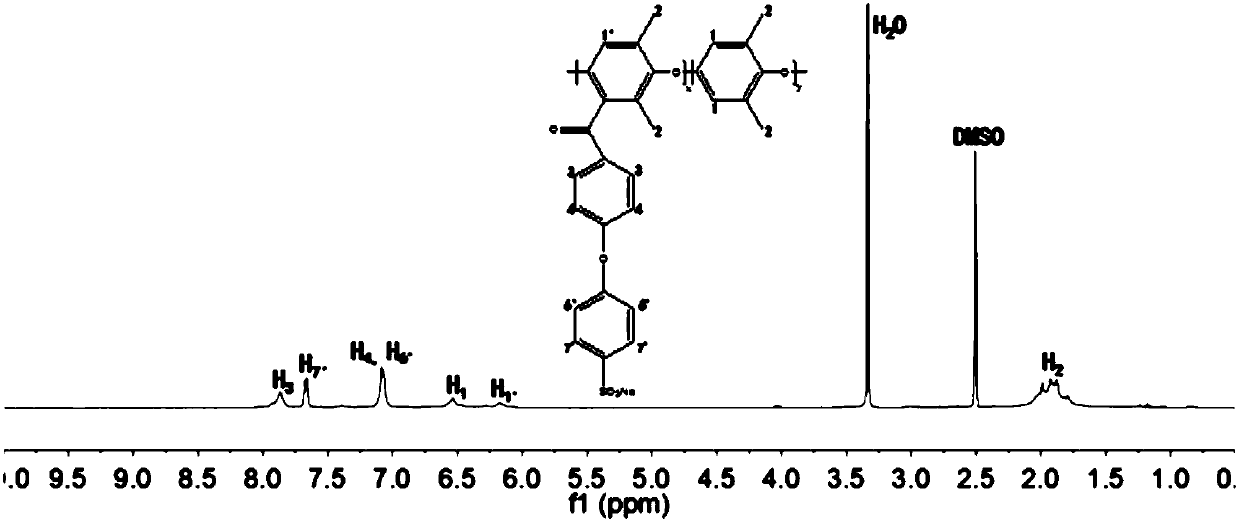
[0032] (2) Condensation reaction of the acylation product: the N-methylpyrrolidone solution of the acylation product with a w / v concentration of 10% is prepared, and the degree of acylation is 70%; under the protection of an inert gas, add 2.25 times of acylation The amount of sodium p-hydroxybenzenesulfonate in the amount of the product substance; then add 5.06 times the amount of potassium carbonate in the amount of the acylation product substance, remove water by bubbling...
Embodiment 2
[0034] (1) Acylation of polyphenylene ether: Prepare a 1,2-dichloroethane solution of polyphenylene ether with a w / v concentration of 0.06%, add 0.7 times of polyphenylene ether under the protection of an ice-water bath and an inert gas aluminum trichloride in the amount of substance, and then add p-fluorobenzoyl chloride 0.6 times the amount of polyphenylene ether; move it to an oil bath, and react with magnetic stirring at 60°C for 10h; pour it into ethanol to precipitate, and wash repeatedly. and dry;
[0035] (2) Condensation reaction of acylated products: N,N-dimethylacetamide solution of acylated products with a w / v concentration of 12% is prepared, the degree of acylation is 60%; under the protection of an inert gas, add 2.25 twice the amount of the acylation product; add potassium carbonate three times the amount of the acylation product, remove water by bubbling, and react at 140°C for 110 hours; pour into ethyl acetate to precipitate , repeated cleaning, and drying;...
Embodiment 3
[0037] (1) Acylation of polyphenylene ether: Prepare a 1,2-dichloroethane solution of polyphenylene ether with a w / v concentration of 0.06%, add 0.6 times of polyphenylene ether under the protection of an ice-water bath and an inert gas aluminum trichloride in the amount of substance, and then add p-fluorobenzoyl chloride 0.5 times the amount of polyphenylene ether; move it to an oil bath, and react with magnetic stirring at 60°C for 10 hours; pour it into ethanol to precipitate, and wash repeatedly. and dry;
[0038] (2) Condensation reaction of acylated products: N,N-dimethylacetamide solution of acylated products with a w / v concentration of 12% is prepared, the degree of acylation is 50%; under the protection of an inert gas, add 2.25 twice the amount of the acylation product as sodium p-hydroxybenzenesulfonate; then add 3 times the amount of potassium carbonate as the acylation product, remove water by bubbling, and react at 140°C for 92 hours; pour into ethyl acetate to p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com