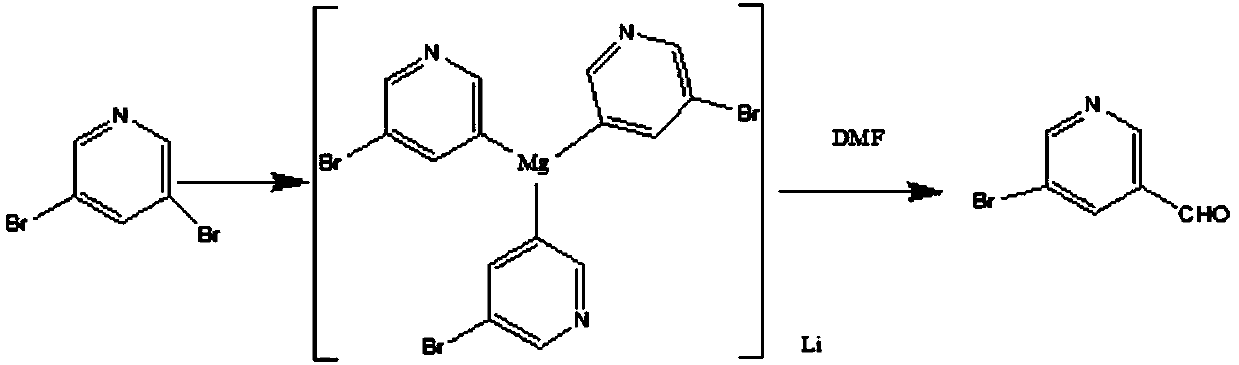
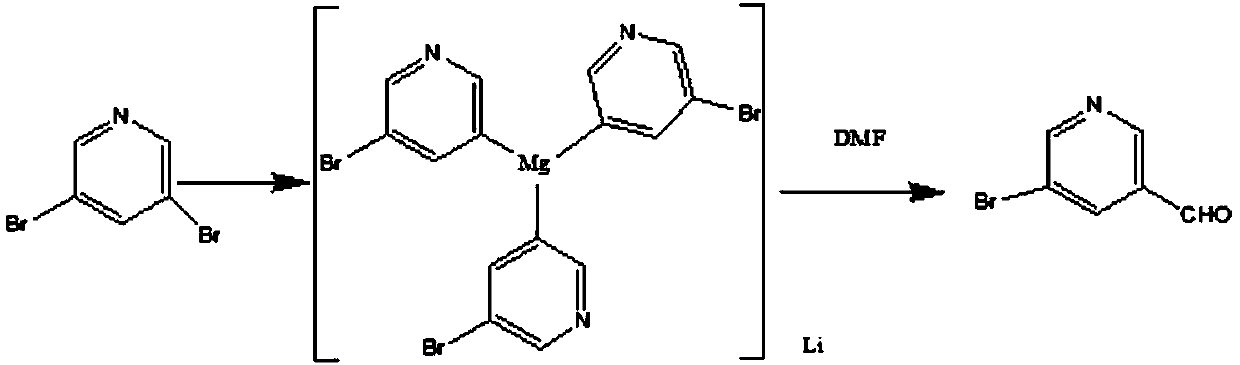
Method for synthesizing 5-bromopyridine-3-formaldehyde
A synthetic method, bromopyridine technology, applied in the direction of organic chemistry, can solve the problems of harsh reaction conditions, difficult post-processing, difficult industrialization, etc., and achieve the effect of easy operation, less steps, and energy saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Take 250g of 3,5-dibromopyridine, 1000ml of tetrahydrofuran, and 150g of tetramethylethylenediamine, put them into a 5L reaction bottle, and start stirring. The system was cooled to 10-15°C in an ice-water bath. Take 750ml of isopropylmagnesium chloride (2.6M, THF) and drop it into the reaction solution, keeping the temperature below 15°C (warming up slightly). After dropping, the ice-water bath was removed and the reaction was maintained at 20-25°C for 1-2h. The reaction solution was cooled to 5-10°C with an ice-water bath. Take 130g of DMF and dissolve it in 100ml of THF. Slowly add the DMF-THF mixture into the reaction solution dropwise, keeping the internal temperature below 15°C (exothermic, should be added dropwise). After dropping, keep the reaction at 10-15°C for 30min. Pour the reaction solution into 2L of ice water, stir for 10 min., let stand, separate the liquid, and collect the aqueous phase and the organic phase respectively. The aqueous phase was ext...
Embodiment 2
[0023] Same as Example 1, only the
[0024] The volume ratio of 3,5-dibromopyridine to tetrahydrofuran is changed to: 1:3
[0025] The mass ratio of 3,5-dibromopyridine to tetramethylethylenediamine was changed to 1:0.5;
[0026] The molar ratio of 3,5-dibromopyridine, isopropylmagnesium chloride, and DMF was changed to 1:1.2-:1.5;
[0027] The volume of ice water is 1.5 times the volume of tetrahydrofuran in the reaction solution.
[0028] The yield of the target product was 65.4%.
Embodiment 3
[0030] Same as Example 1, only the
[0031] The volume ratio of 3,5-dibromopyridine to tetrahydrofuran was changed to: 1:6
[0032] The mass ratio of 3,5-dibromopyridine to tetramethylethylenediamine was changed to 1:1;
[0033] The molar ratio of 3,5-dibromopyridine, isopropylmagnesium chloride, and DMF was changed to 1:1.5:2;
[0034] The volume of ice water is 4 times the volume of tetrahydrofuran in the reaction solution.
[0035] The yield of the target product was 66.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com