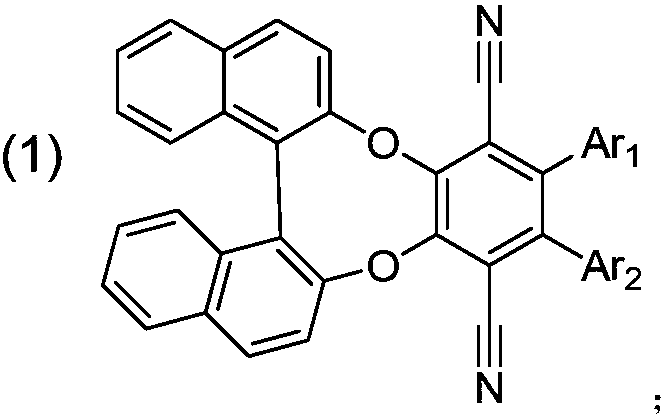
Asymmetric heat-activated delayed fluorescent material, synthesis method and application thereof
A technology of thermally activated delayed and fluorescent materials, applied in luminescent materials, chemical instruments and methods, electrical components, etc., can solve the problems of no circularly polarized luminescence, energy loss, etc., to achieve efficient and stable light emission, high yield, synthesis Method and purification process simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
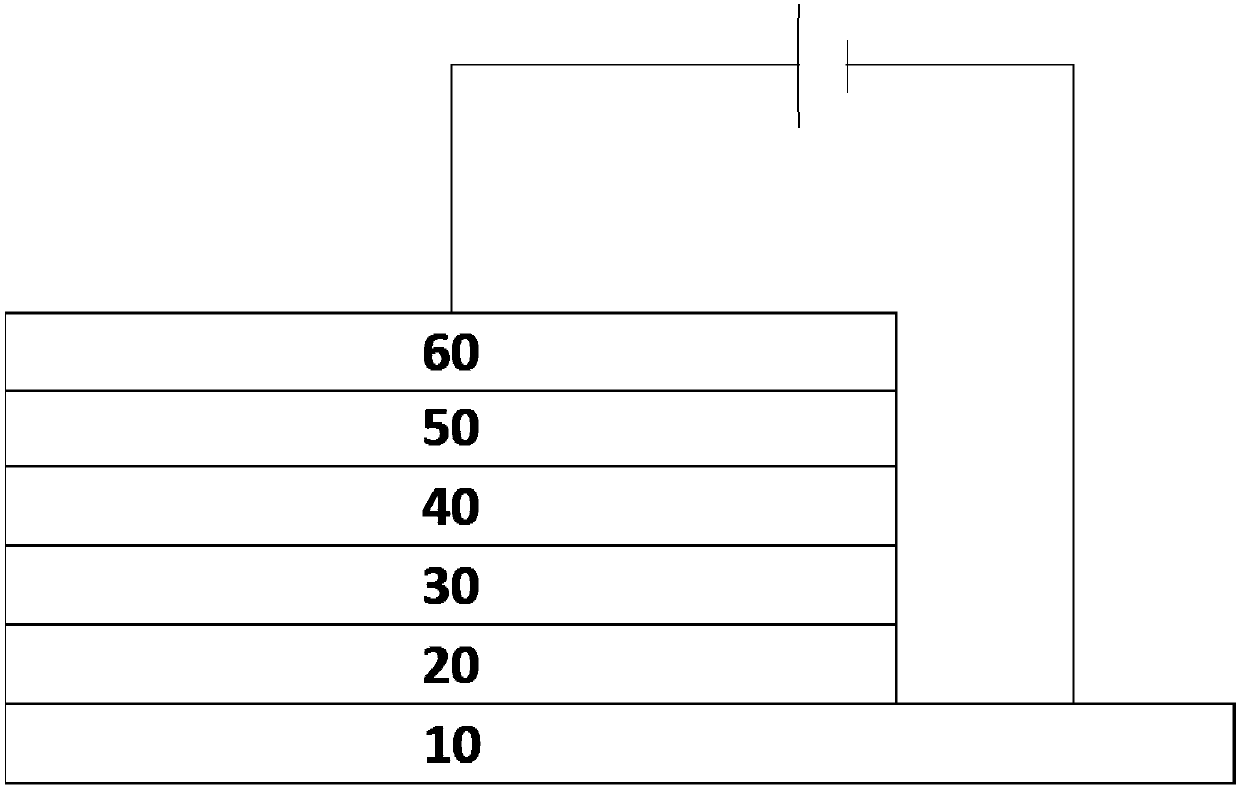
Image
Examples
Embodiment 1
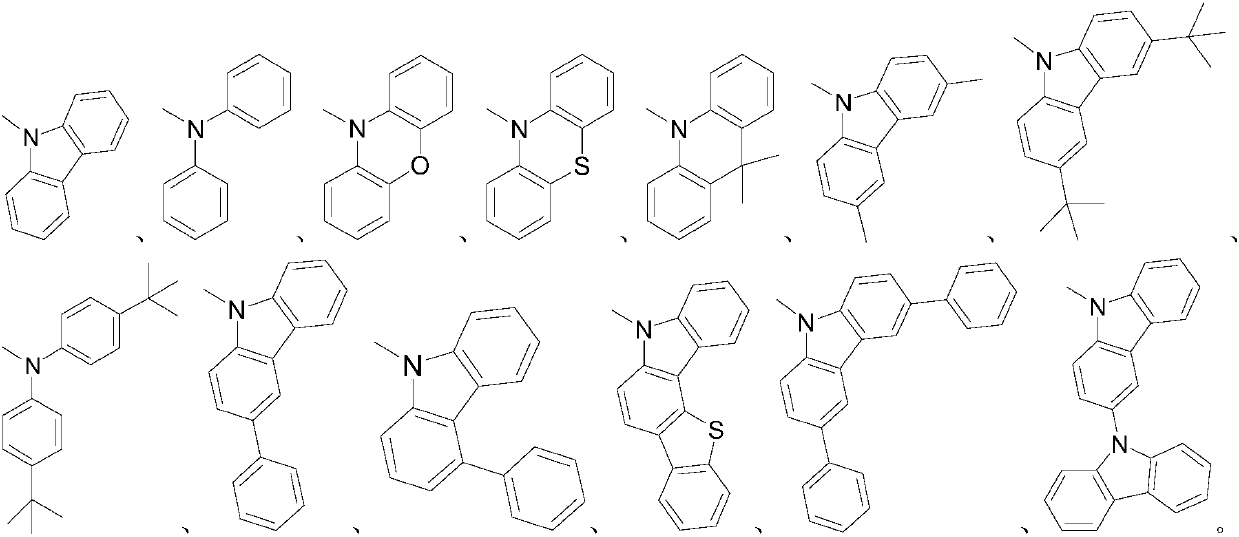
[0048] (R)-2-(9H-carbazol-9-yl)-3-(9,9-dimethylacridin-10(9H)-yl)benzo[b]binaphtho[2,1- e: Synthesis of 1',2'-g][1,4]dioxaoctatriene-1,4-dicarbonitrile, the synthetic route is as follows:
[0049]
[0050] Under argon protection, tetrafluoroterephthalonitrile (0.20g, 1.00mmol) and (R)-1,1'-bi-2-naphthol (0.286g, 1.00mmol) were added into a three-necked flask, and Dissolve in 10mL DMF, add K 2 CO 3 (0.28g, 2.00mmol), stirred the reaction at room temperature for 12 hours; then added 9,10-dihydro-9,9-dimethylacridine (0.21g, 1.00mmol) and potassium carbonate (0.14g, 1.00 mmol), stirred and reacted at 80°C for 12 hours; after the reaction solution was cooled to room temperature, 9H-carbazole (0.21g, 1.25mmol) and potassium carbonate (0.17g, 1.25mmol) were added, and the stirred reaction was continued at room temperature for 8 hours . After the reaction was finished, the reaction solution was poured into 150 mL of saturated brine to precipitate solids, and suction filtered. ...
Embodiment 2
[0052] (S)-2-(9H-carbazol-9-yl)-3-(9,9-dimethylacridin-10(9H)-yl)benzo[b]binaphtho[2,1- e: Synthesis of 1',2'-g][1,4]dioxaoctatriene-1,4-dicarbonitrile, the synthetic route is shown in the following formula:
[0053]
[0054] Under argon protection, tetrafluoroterephthalonitrile (0.20g, 1.00mmol) and (S)-1,1'-bi-2-naphthol (0.286g, 1.00mmol) were added into the three-necked flask, and Dissolve in 10mL DMF, add K 2 CO 3 (0.28g, 2.00mmol), stirred the reaction at room temperature for 12 hours; then added 9,10-dihydro-9,9-dimethylacridine (0.21g, 1.00mmol) and potassium carbonate (0.14g, 1.00 mmol), stirred and reacted at 80°C for 12 hours; after the reaction solution was cooled to room temperature, 9H-carbazole (0.21g, 1.25mmol) and potassium carbonate (0.17g, 1.25mmol) were added, and the stirred reaction was continued at room temperature for 8 hours . After the reaction was finished, the reaction solution was poured into 150 mL of saturated brine to precipitate solids, ...
Embodiment 3
[0056] (R)-2-(9H-carbazol-9-yl)-3-(10H-phenothiazin-10-yl)benzo[b]dinaphtho[2,1-e:1',2' -g] [1,4] The synthesis of dioxaoctatriene-1,4-dicarbonitrile, the synthetic route is as follows:
[0057]
[0058] Under argon protection, tetrafluoroterephthalonitrile (0.30g, 1.50mmol) and (R)-1,1'-bi-2-naphthol (0.43g, 1.50mmol) were added into a three-necked flask, and Dissolve in 15mL DMF, add K 2 CO 3 (0.42g, 3.00mmol), stirred and reacted at room temperature for 12 hours; then added 10H-phenothiazine (0.30g, 1.50mmol) and potassium carbonate (0.21g, 1.50mmol), stirred and reacted at room temperature for 12 hours; Finally, 9H-carbazole (0.31 g, 1.88 mmol) and potassium carbonate (0.26 g, 1.88 mmol) were added, and the stirring reaction was continued at room temperature for 8 hours. After the reaction, the reaction solution was poured into 200mL saturated brine to precipitate solids, and suction filtered, and the obtained crude product was separated and purified by silica gel co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com