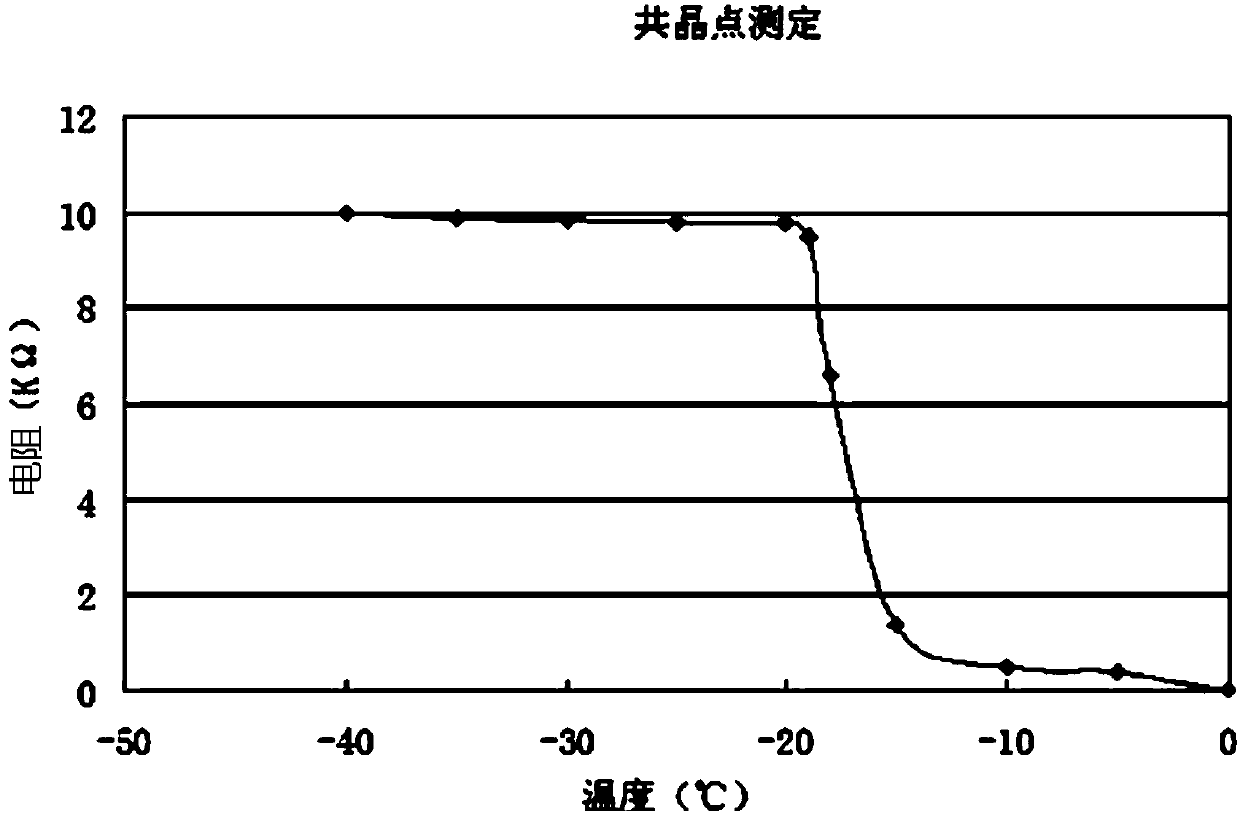
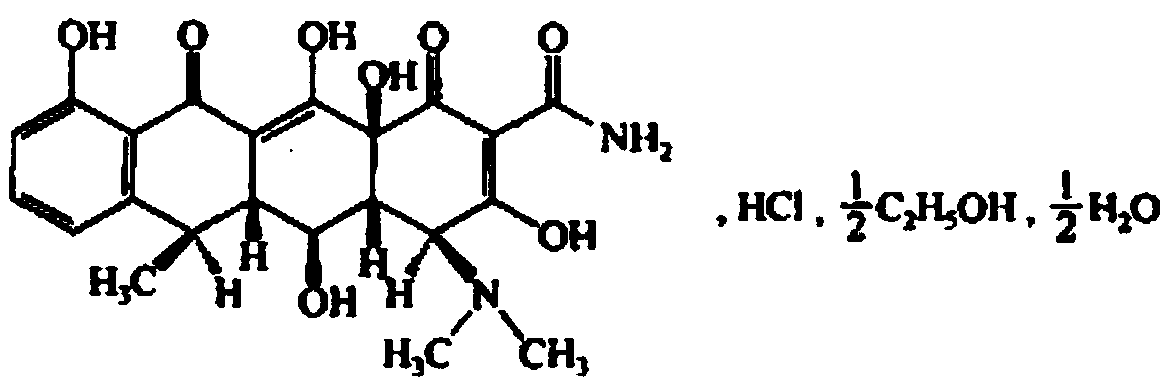
Injection doxycycline hyclate freeze-drying powder and preparation method thereof
A technology of doxycycline hydrochloride and freeze-dried powder, which is applied in the field of doxycycline hydrochloride freeze-dried powder for injection and its preparation, can solve the problems of the increase of related substances, failure to shape, low analysis and drying temperature, etc., and achieve relevant The effect of small substance content, prolonging the service life, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Prescription (1000 sticks): specification 0.1g (in C 22 h 24 N 2 o 8 count)
[0037] Element
Dosage
Doxycycline hydrochloride (in C 22 h 24 N 2 o 8 count)
100g
Vitamin C
400g
cysteine hydrochloride
5g
lactose
300g
hydrochloric acid or sodium hydroxide
Appropriate amount
Water for Injection
Add to 3500ml
[0038] Preparation:
[0039] (1) Dosing: inject 2625ml of water for injection into the dosing tank, control the temperature at 20°C, add 400g of vitamin C, 5g of cysteine hydrochloride, stir to dissolve; add 300g of lactose, 100g of doxycycline hydrochloride in turn, stir Make it dissolve, adjust the pH to 1.8 with sodium hydroxide or hydrochloric acid, add 0.03% (w / v) activated carbon to the liquid medicine, stir at room temperature for 15 minutes, add water for injection to 3500ml, titanium rod filter (specification: 1.0μm material : Titanium rod) filtration cycle for 5-1...
Embodiment 2
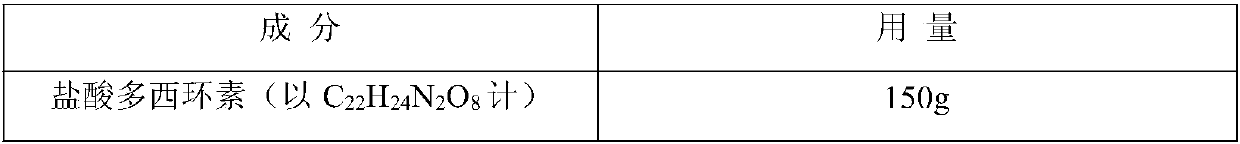
[0049] Prescription (1000 sticks): specification 0.15g (in C 22 h 24 N 2 o 8 count)
[0050]
[0051]
[0052] Preparation:
[0053] (1) Dosing: Inject 2625ml of water for injection into the dosing tank, control the temperature at 25°C, add 500g of vitamin C, 8g of cysteine hydrochloride, stir to dissolve; add 350g of lactose, 150g of doxycycline hydrochloride in turn, and stir Make it dissolve, adjust the pH to 2.2 with sodium hydroxide or hydrochloric acid, add 0.03% (w / v) activated carbon to the liquid medicine, stir at room temperature for 15 minutes, add water for injection to 3500ml, and use a titanium rod filter (specification: 1.0μm material : Titanium rod) filtration cycle for 5-10 minutes for decarburization. Pass the liquid medicine through the pre-filter (specification: 0.45μm material: polyethersulfone), secondary filter (specification: 0.22μm material: polyethersulfone), terminal sterilization filter (specification: 0.22μm material) within 30 minutes...
Embodiment 3
[0058] Prescription (1000 sticks): specification 0.2g (in C 22 h 24 N 2 o 8 count)
[0059] Element
Dosage
Doxycycline hydrochloride (in C 22 h 24 N 2 o 8 count)
200g
Vitamin C
600g
cysteine hydrochloride
10g
lactose
400g
hydrochloric acid or sodium hydroxide
Appropriate amount
Water for Injection
Add to 3500ml
[0060] Preparation:
[0061] (1) Dosing: Inject 2625ml of water for injection into the dosing tank, control the temperature at 30°C, add 600g of vitamin C, 10g of cysteine hydrochloride, stir to dissolve; add 400g of lactose, 200g of doxycycline hydrochloride in turn, and stir Make it dissolve, adjust the pH to 3.3 with sodium hydroxide or hydrochloric acid, add 0.03% (w / v) activated carbon to the liquid medicine, stir at room temperature for 15 minutes, add water for injection to 3500ml, titanium rod filter (specification: 1.0μm material : Titanium rod) filtration cycle f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com