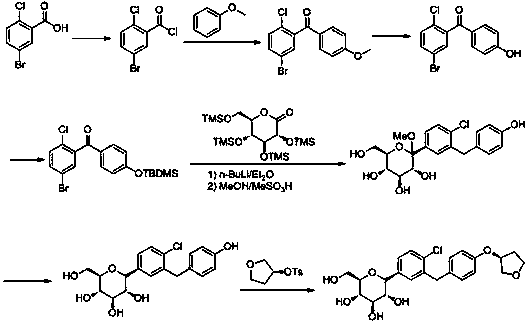
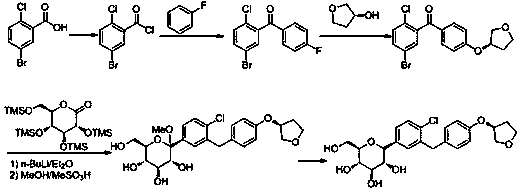
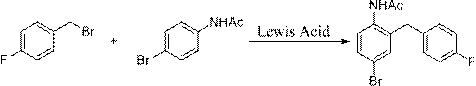Synthesis process for empagliflozin
A technology of empagliflozin and synthesis process, applied in the field of medicinal chemistry, can solve the problems of high cost and expensive raw materials, and achieve the effects of less impurities, improved purity and easy availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] (1) Preparation of p-bromoacetanilide (I): Dissolve p-bromoacetaniline in water, add dropwise methanol solution of acetic anhydride under ice bath, after the dropwise addition, react at room temperature for 3-5h to prepare p-bromoacetanilide ( I); Concrete reaction is as follows:
[0046]
[0047] (2) Preparation of p-fluorobenzyl bromide (II): Dissolve 4-fluorotoluene and NBS in a solvent, and prepare p-fluorobenzyl bromide (II) in the presence of an initiator; among them, the solvent can be carbon tetrachloride or chloroform Any one of them, preferred toxicity is weaker chloroform as solvent; Initiator can select any one in BPO or AIBN, preferred toxicity is weaker BPO; Concrete reaction is as follows:
[0048]
[0049] (3) Preparation of 4-bromo-2-(4-fluorobenzyl)acetanilide (III): Dissolve p-fluorobenzyl bromide and p-bromoacetanilide in a solvent, catalyzed by Lewis acid, and synthesize 4 -Bromo-2-(4-fluorobenzyl)acetanilide (III); wherein, the solvent can b...
Embodiment 1
[0062] The preparation of embodiment 1 p-bromoacetanilide (I)
[0063] Take 171kg of p-bromoaniline, dissolve it in 300kg of water, stir in ice bath for 30min, add dropwise the methanol solution of acetic anhydride (102kg of acetic anhydride + methanol 200kg), drop it in 30min, after the dropwise addition, stir in ice bath for 1h, then raise the temperature to Continue to react at room temperature for 2 h. After the reaction, filter, wash the filter cake until neutral, and dry to obtain 210 kg of light yellow solid, with a yield of 99%.
Embodiment 2
[0064] Embodiment 2 Preparation of p-fluorobenzyl bromide (II)
[0065] Take 110kg of 4-fluorotoluene, dissolve it in 500kg of chloroform, add 180kg of NBS, add 12kg of BPO, heat up to 70°C for reflux reaction, and react for 6h. After the reaction, cool to room temperature, filter, and recover chloroform from the filtrate under reduced pressure, and distill the residue under reduced pressure 177kg of light yellow liquid was obtained, with a yield of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com