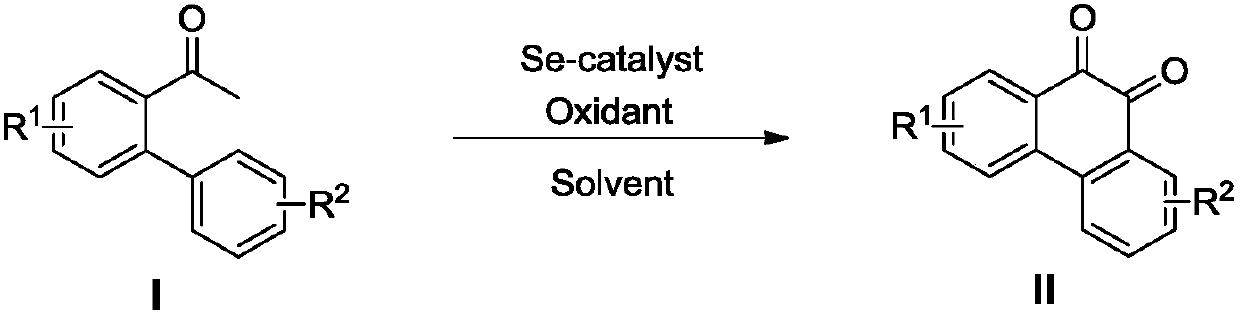
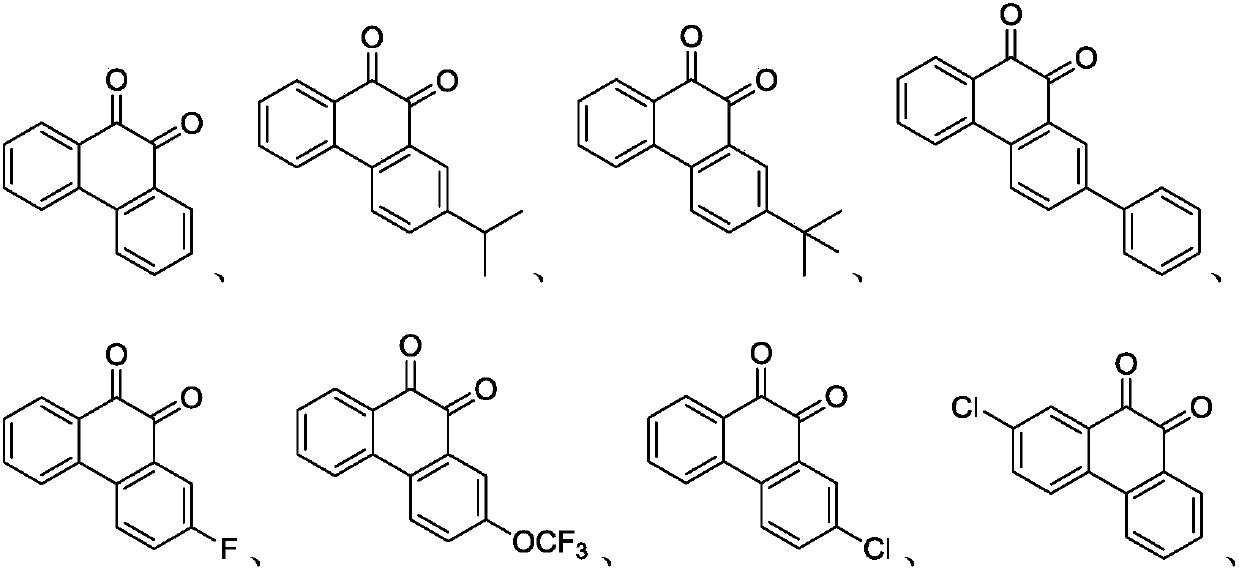
Preparation method of phenanthrenequinone and derivatives of phenanthrenequinone
A derivative, the technology of phenanthrenequinone, which is applied in the field of synthesis of phenanthrenequinone and its derivatives, can solve the problems of harsh reaction conditions, harmful oxidants, complicated operation, etc., and achieve the effects of mild reaction temperature, simple operation and simple reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: take o-phenylacetophenone as raw material, prepare phenanthrenequinone
[0026]
[0027] Add 58.8mg (0.30mmol) of o-phenylacetophenone, 2.4mg (0.03mmol) of selenium powder, and 318.6mg (0.9mmol) of Selectfluor into a 25mL pressure-resistant sealed container in sequence, and then add 3mL of acetonitrile. The mixture was stirred and reacted at 80°C, followed by TLC detection, and the reaction was completed after 24 hours. The reaction solution was diluted with 10 mL of dichloromethane, and the clear liquid was obtained by filtration. After the organic solvent was evaporated, it was purified with a silica gel column, and then column chromatography (Eluant ratio: volume ratio of petroleum ether to ethyl acetate 10:1) Separation, collecting the eluate and evaporating the solvent to obtain 48 mg of reddish-brown solid phenanthrenequinone (76% yield).
[0028] Reddish-brown solid; m.p.218–220°C; IR(neat):1673(C=O)cm -1 ; 1 H NMR (400MHz, CDCl 3 ):δ8.16(dd,J...
Embodiment 2
[0029] Embodiment 2: take o-phenylacetophenone as raw material, prepare phenanthrenequinone
[0030]
[0031] Add 58.8mg (0.30mmol) of o-phenylacetophenone, 4.8mg (0.06mmol) of selenium powder, and 159.3mg (0.45mmol) of Selectfluor into a 25mL pressure-resistant sealed container in sequence, and then add 1.5mL of nitromethane. The mixture was stirred and reacted at 100°C, followed by TLC detection, and the reaction was completed after 24 hours. The reaction liquid was diluted with 10 mL of dichloromethane, and the clear liquid was obtained by filtration. After the organic solvent was evaporated, it was purified with a silica gel column, and then column chromatography (Eluant ratio: volume ratio of petroleum ether to ethyl acetate 10:1) Separation, collecting the eluate and evaporating the solvent to obtain 40 mg of reddish-brown solid phenanthrenequinone (65% yield).
[0032] Reddish-brown solid; m.p.218–220°C; IR(neat):1673(C=O)cm -1 ; 1 H NMR (400MHz, CDCl 3 ):δ8.16(dd...
Embodiment 3
[0033] Embodiment 3: take o-phenylacetophenone as raw material, prepare phenanthrenequinone
[0034]
[0035]Add 58.8mg (0.30mmol) of o-phenylacetophenone, 1.2mg (0.015mmol) of selenium powder, and 371.7mg (1.05mmol) of Selectfluor into a 25mL pressure-resistant sealed container, and then add 4.5mL of acetonitrile. The mixture was stirred and reacted at 100°C, followed by TLC detection, and the reaction was completed after 12 hours. The reaction solution was diluted with 10 mL of dichloromethane, and the clear liquid was obtained by filtration. After the organic solvent was evaporated, it was purified with a silica gel column, and then column chromatography (Eluant ratio: volume ratio of petroleum ether to ethyl acetate 10:1) separation, collecting the eluate and evaporating the solvent to obtain 44 mg of reddish-brown solid phenanthrenequinone (70% yield).
[0036] Reddish-brown solid; m.p.218–220°C; IR(neat):1673(C=O)cm -1 ; 1 H NMR (400MHz, CDCl 3 ):δ8.16(dd,J 1 =8.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com