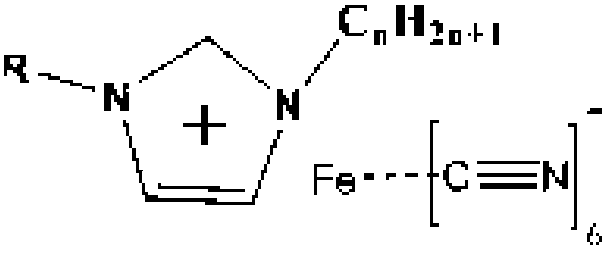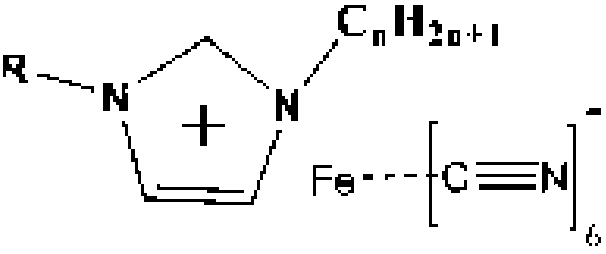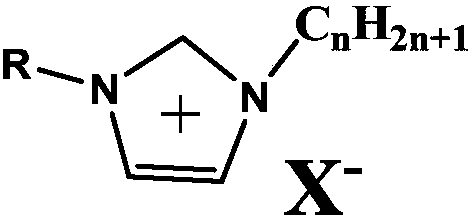Iron-based cyanogroup-containing anion imidazole ionic liquid catalyst, and preparation method and applications thereof
The technology of imidazole ionic liquid and catalyst is applied in the field of catalysis, which can solve the problems of unrecyclable catalyst, unstable storage and high catalyst cost, and achieve the effects of low cost of preparing raw materials, good product distribution and high utilization rate of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Iron-based cyano-containing anion imidazole ionic liquid catalyst A (0.2mmol), join in the there-necked flask with magnetic stirring heating device and condensation device, under the protection of nitrogen, drop 1-hexene (0.6mol) successively through dropping funnel ), slowly warming up to a reaction temperature of 65°C. Then add trimethoxyhydrogensilane (0.8mol) dropwise to the liquid catalyst system, turn on condensing reflux, keep the reaction temperature at 65°C, continue to stir the reaction for 6h, stop the reaction, let the ionic liquid stand to cool below room temperature to solidify, separate the product and ionic liquid. Corresponding fractions were collected by distillation under reduced pressure. The conversion rate of 1-hexene was determined by GC-MS to be 98.9%, and the yield of β-addition product (hexyltrimethoxysilane) was 97.5%.
Embodiment 2
[0037] Iron-based cyano-containing anion imidazole ionic liquid B (0.2mmol), was added to a three-necked flask with a magnetic stirring heating device and a condensation device, and under nitrogen protection, 1-heptene (0.6mol) was sequentially added dropwise through a dropping funnel , slowly warming up to 85°C. Then add trimethoxyhydrogensilane (0.7mol) dropwise to the liquid catalyst system, keep the reaction temperature at 85°C, turn on the condensing reflux, continue to stir the reaction for 6h, stop the reaction, let it cool down to below room temperature and the ionic liquid solidifies, separate the product and ionic liquid. Corresponding fractions were collected by distillation under reduced pressure. The conversion rate of 1-heptene was determined by GC-MS to be 96.9%, and the yield of β-addition product (heptyltrimethoxysilane) was 94.5%.
Embodiment 3
[0039]Iron-based cyano-containing anion imidazole ionic liquid C (0.1mmol), was added to a three-necked flask with a magnetic stirring heating device and a condensation device, and under nitrogen protection, 1-undecene (0.3mol ), slowly warming up to a reaction temperature of 85°C. Then add trimethoxyhydrogensilane (0.5mol) dropwise to the liquid catalyst system, keep the reaction temperature at 85°C, turn on the condensing reflux, continue to stir the reaction for 6h, stop the reaction, let the ionic liquid stand to cool below room temperature to solidify, separate the product and ionic liquid. Corresponding fractions were collected by distillation under reduced pressure, and the conversion rate of 1-undecene was determined to be 88.9% by GC-MS, and the yield of β-addition product (undecyltrimethoxysilane) was 85.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com