Recombinant proteins PspA1, PspA2 and PspA3, and polysaccharide conjugation vaccine containing the same
A technology combining vaccines and recombinant proteins, applied in the field of recombinant proteins PspA1, PspA2 and PspA3 and polysaccharide conjugate vaccines containing them, can solve problems such as affecting immune effect, increasing tetanus toxoid inoculation, overloading or immunosuppression, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1. Codon optimization and gene synthesis of the surface protein A gene sequence of Streptococcus pneumoniae
[0039] The gene sequence was synthesized by Zhongmeitaihe Biotechnology Co., Ltd., connected with the expression plasmid pET30a and transformed into E. coli expression strain BL21 (DE3). The gene sequence length is 1143bp for PspA1, 1122bp for PspA2, and 1431bp for PspA3. According to the gene sequence and amino acid sequence, the surface protein A of Streptococcus pneumoniae was artificially synthesized by means of codon optimization. The three branches synthesized were from strains DBL6A, RX1 and EF3296 (GenBank, sequence numbers were: AF071805.1, M74122.1 and AF071816), respectively, which were named PspA1, PspA2 and PspA3, and the signal peptide was removed, using The GENE designer software optimizes the codons of the selected gene sequence, and optimizes the rare codons into codons suitable for expression in the E. coli expression system to increas...
Embodiment 2
[0040] Example 2. Obtaining recombinant plasmids pET30a-PspA1, pET30a-PspA2 and pET30a-PspA3
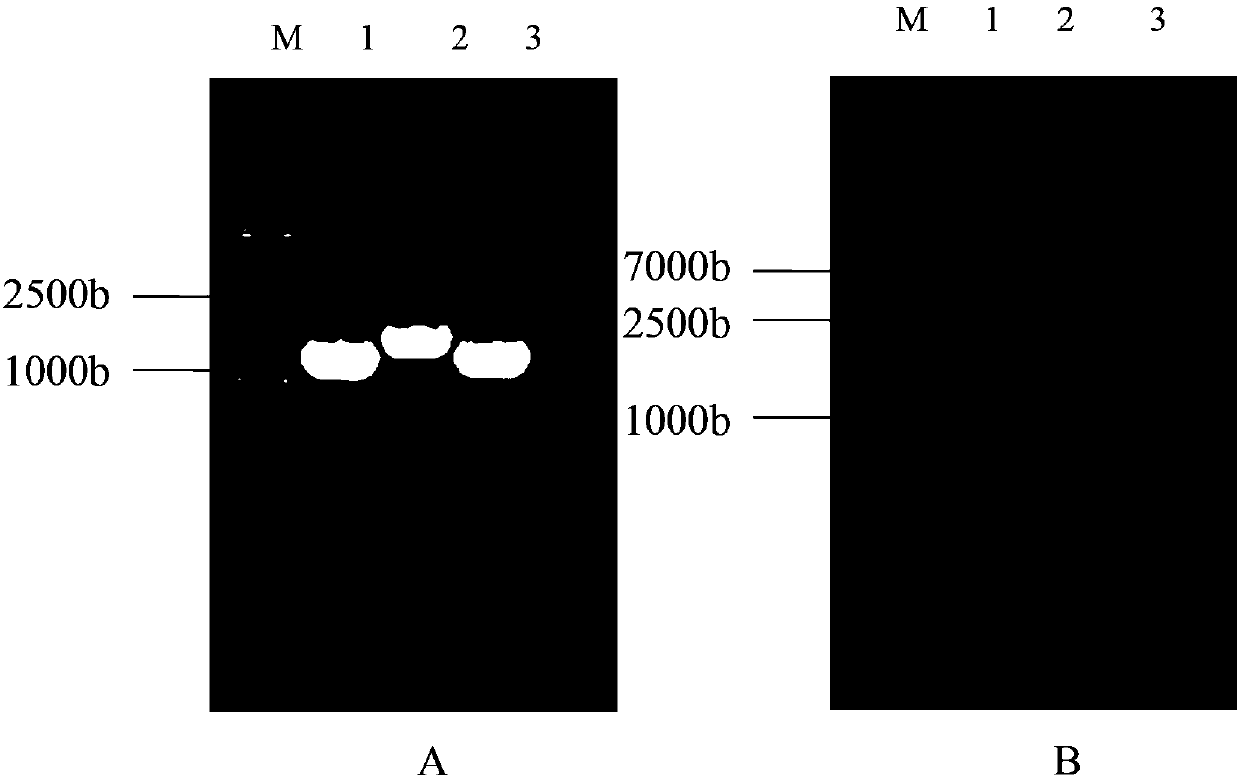
[0041] The gene sequence was synthesized by Zhongmeitaihe Biotechnology Co., Ltd., and the target fragments of the three different branched proteins of PspA were cloned into the pET-30a(+) expression vector and transformed into the E. coli expression strain BL21(DE3). The transformed bacterial liquid was inoculated into LB liquid medium containing kanamycin, and the plasmid was extracted after shaking culture at 37°C for 12 hours.
[0042] In order to extract the plasmid, collect the precipitate of 1.5-3ml bacterial solution in a 1.5ml Eppendorf centrifuge tube, add 100μL of solution 1, shake until fully suspended. Add 150 μL of solution 2, and immediately invert the centrifuge tube gently several times to fully lyse the bacteria, and the lysed bacteria suspension becomes clear. The tubes were then placed on ice for 1-2 minutes. Add 150 μL of solution 3, immediately invert the cent...
Embodiment 3
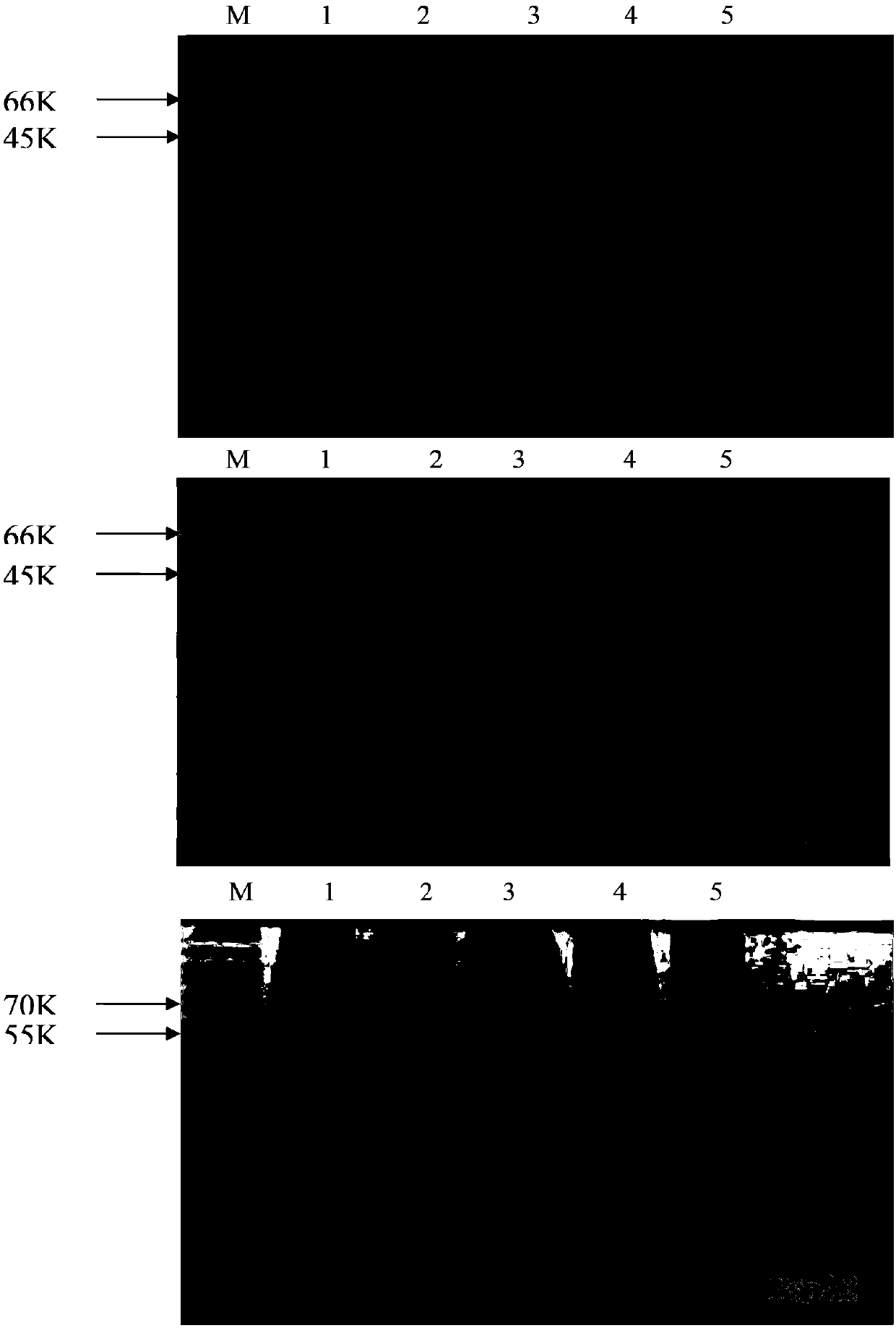
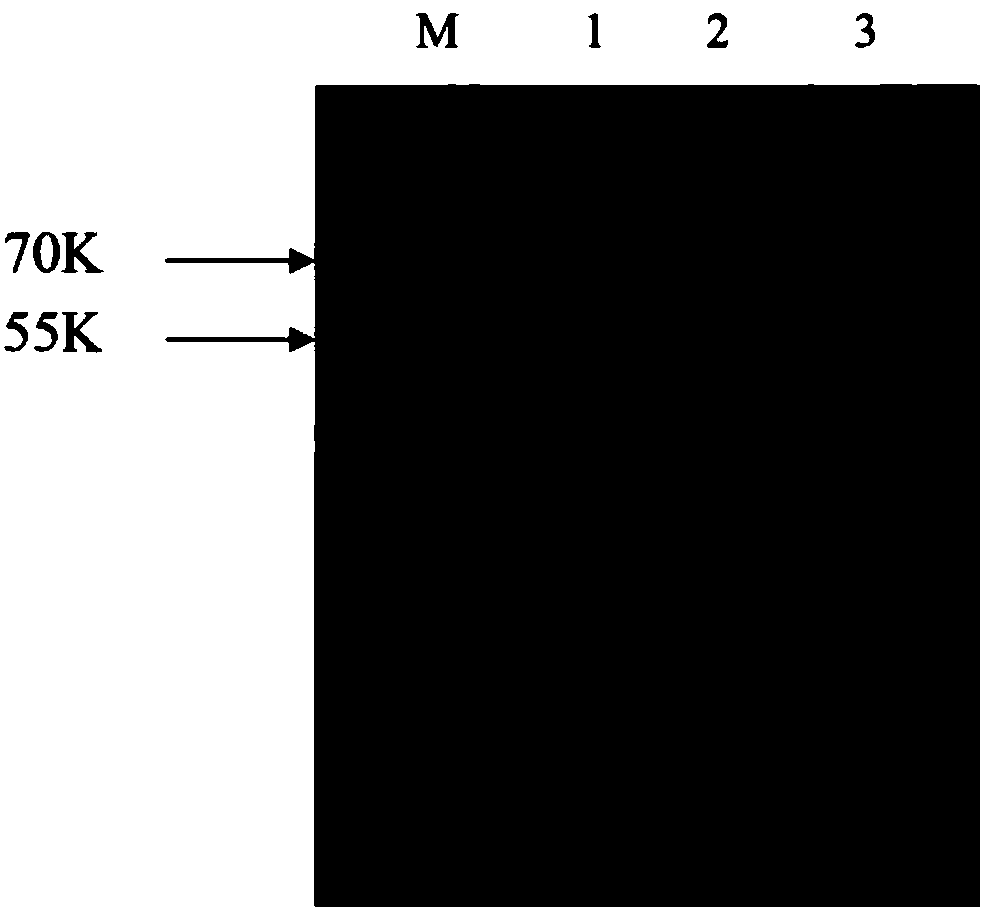
[0051] Example 3. Induced expression of recombinant PspA1, PspA2 and PspA3 proteins in Escherichia coli BL21 (DE3)
[0052] Take 50 μL of the correct identified Escherichia coli BL21 (DE3) bacterial solution, inoculate it in 5 ml of LB liquid medium containing kanamycin (50 μg / ml), and cultivate overnight at 37° C. with shaking. Transfer to fresh LB liquid medium containing kanamycin (50 μg / ml) at a ratio of 1:50, culture at 37°C with shaking (200 rpm) until the 550nm OD value reaches 0.5-1.0. Add IPTG to a final concentration of 1 mM, and induce expression at 37°C for 4hs. Take 1ml of bacterial liquid, centrifuge at 5000rpm at 4°C for 5min, and collect the bacterial cells. The bacterial pellet was suspended in 100 μL 1xSDS loading buffer (50 mM Tris-Cl pH6.8, 100 mM DTT, 2% SDS, 0.1% bromophenol blue, 10% glycerol), and analyzed by SDS-PAGE electrophoresis.
[0053] According to "Molecular Cloning", prepare 12% separating gel and 5% stacking gel, cast the gel, and prepare e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com