Method for synthesizing aromatic alkyl ether
An aryl alkyl ether and aromatic phenol technology, which is applied in the synthesis field of aryl alkyl ether, can solve the problems of unavoidable hydrolysis of dimethyl sulfate, low activity of reactants, low production efficiency and the like, and achieves high production efficiency, High reaction rate and energy saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
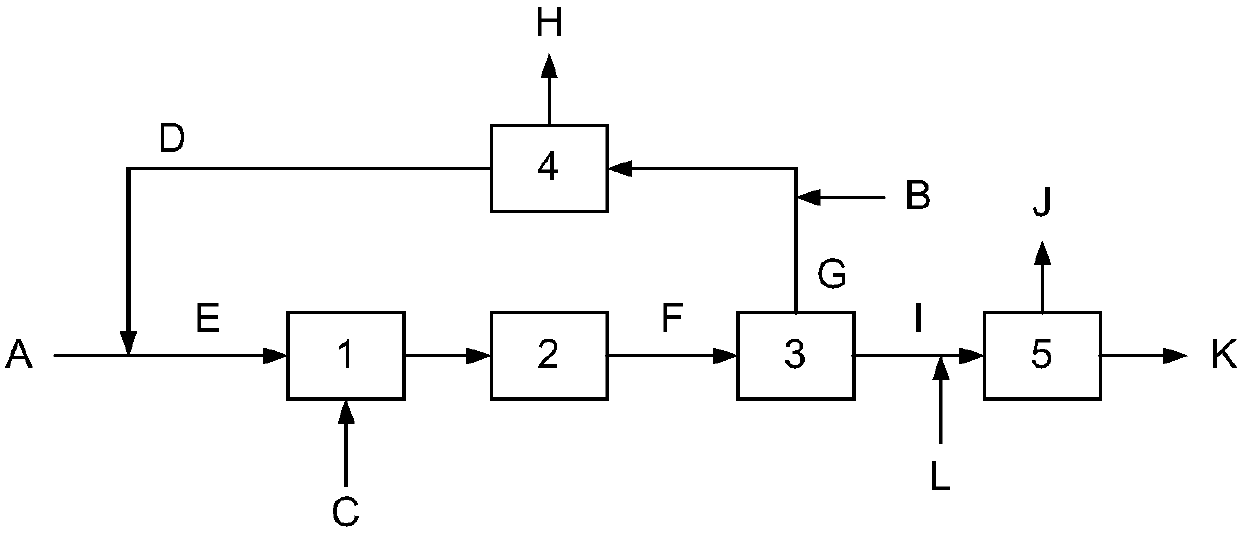
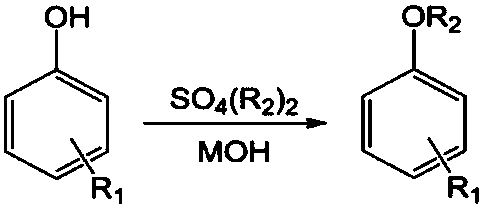
[0021] A method for synthesizing aryl alkyl ethers. The method is based on the principle of high-temperature reaction strengthening. First, the aromatic phenol is reacted with a strong alkali aqueous solution to directly obtain an aromatic phenol salt solution, and then sodium phenate and sulfuric acid are reacted at a high temperature of 80-120°C. The rapid reaction between base esters produces aryl alkyl ethers. The research results show that: the reaction rate of alkyl sulfate etherification and the hydrolysis rate can be increased simultaneously. Under the condition of fast and uniform mixing of reactants, the yield of reaction product is consistent with low temperature reaction. Therefore, in the case of efficient mixing and rapid change of reactor The ideal reaction effect can be obtained under the condition of temperature.
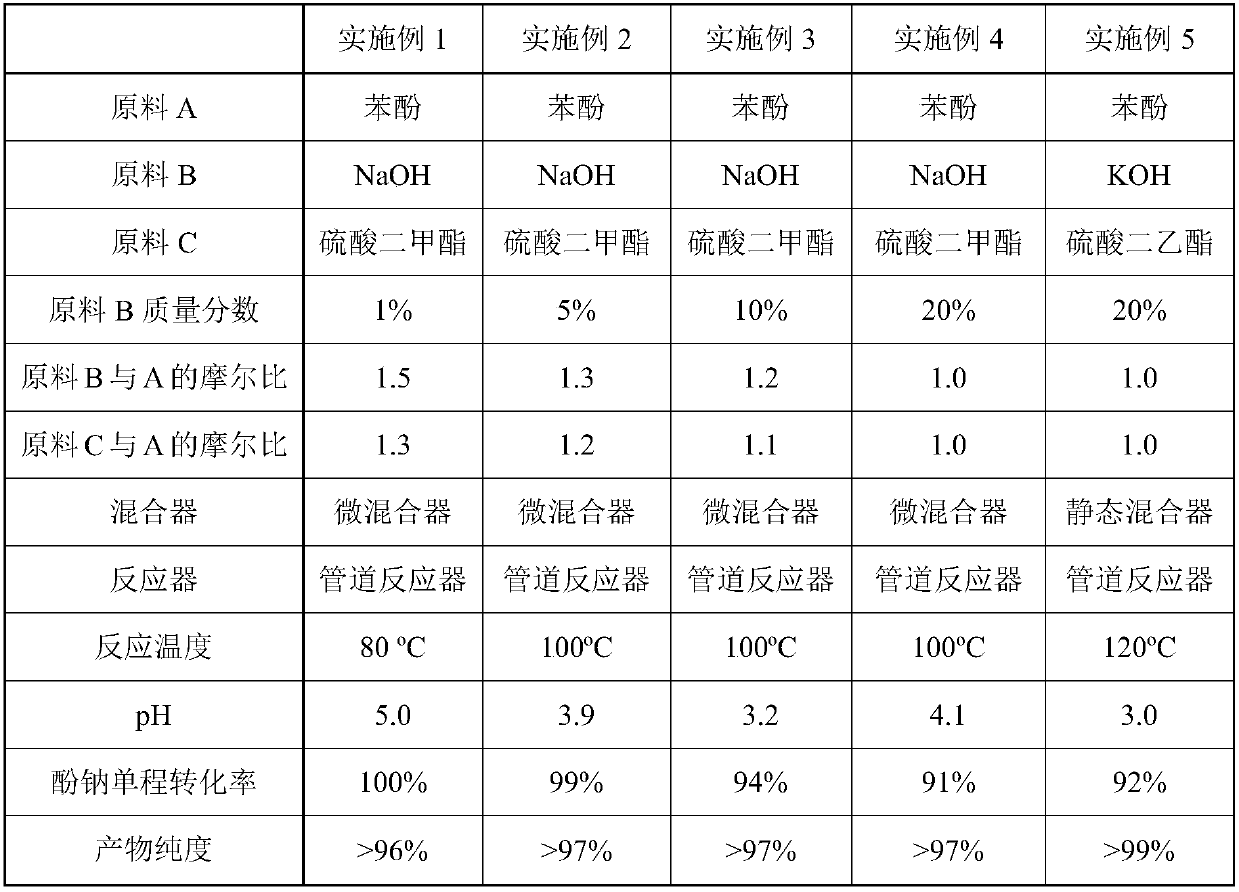
[0022] Below by embodiment and accompanying drawing, the present invention is further described
[0023] The process described in the embodiment i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com