Method for detecting fluoroquinolones medicine based on magnetic nano material purification-carbon quantum dot fluorescence sensibilization
A technology of fluoroquinolones and carbon quantum dots, which is applied in the field of chemical analysis and detection, can solve the problems of serious interference and inability to complete the detection of fluoroquinolones, and achieve the effects of eliminating milk matrix interference, good purification effect, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
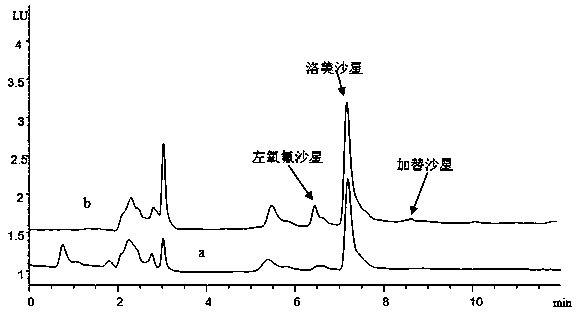
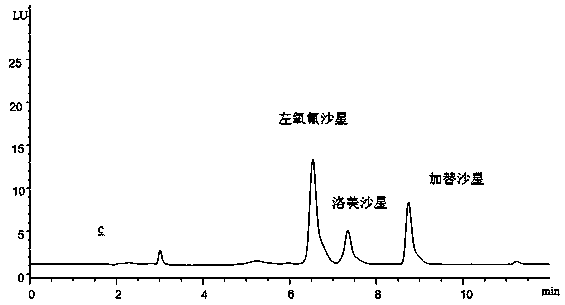
[0025] Embodiment 1: Determination of the residual content of 3 kinds of fluoroquinolones of levofloxacin, lomefloxacin and gatifloxacin in milk, the specific operation steps are as follows:
[0026] (1) Preparation method of water-soluble sulfur-doped fluorescent carbon quantum dots (S-CQDs): Weigh 3.0 g poly(4-styrenesulfonic acid-co-maleic acid) sodium salt, add it to 100 mL ultrapure water , sonicated for 5 min to make it fully mixed to form a colorless transparent liquid, and transferred to a polytetrafluoroethylene reactor, heated at 220 °C for 5 h, cooled to room temperature naturally, and filtered with a filter membrane with a pore size of 0.22 μm. Dialysis was performed for 24 h with a dialysis bag with a molecular weight cut-off of 3500 Da to obtain water-soluble sulfur-doped fluorescent carbon quantum dots, which were stored in a refrigerator at 4 °C in the dark for future use;
[0027] (2) Preparation method of magnetic nanomaterials: Weigh 2.05 g of ferrous ammoni...
Embodiment 2
[0035] Embodiment 2: Determination of the residual content of three kinds of fluoroquinolones in milk, levofloxacin, lomefloxacin and gatifloxacin, the specific operation steps are as follows:
[0036] (1) Preparation method of water-soluble sulfur-doped fluorescent carbon quantum dots (S-CQDs): Weigh 4.0 g poly(4-styrenesulfonic acid-co-maleic acid) sodium salt, add it to 100 mL ultrapure water , ultrasonically for 8 minutes to make it fully mixed to form a colorless transparent liquid, and transferred to a polytetrafluoroethylene reaction kettle, heated at 250 ℃ for 6 hours, cooled to room temperature naturally, filtered with a filter membrane with a pore size of 0.22 μm, and then filtered with a cut-off A dialysis bag with a molecular weight of 3200Da was dialyzed for 24 hours to obtain water-soluble sulfur-doped fluorescent carbon quantum dots, which were stored in a refrigerator at 4 °C in the dark for future use;
[0037] (2) Preparation method of magnetic nanomaterials:...
Embodiment 3
[0041] Embodiment 3: Determination of the residual content of 3 kinds of quinolones in milk, levofloxacin, lomefloxacin and gatifloxacin, the specific operation steps are as follows:
[0042] (1) Preparation method of water-soluble sulfur-doped fluorescent carbon quantum dots (S-CQDs): Weigh 5.0 g poly(4-styrenesulfonic acid-co-maleic acid) sodium salt, add it to 100 mL ultrapure water , ultrasonically for 10 min to make it fully mixed to form a colorless transparent liquid, and transferred to a polytetrafluoroethylene reaction kettle, heated at 230 °C for 7 h, cooled to room temperature naturally, filtered with a filter membrane with a pore size of 0.22 μm, and then filtered with a cut-off A dialysis bag with a molecular weight of 3000Da was dialyzed for 24 hours to obtain water-soluble sulfur-doped fluorescent carbon quantum dots, which were stored in a refrigerator at 4 °C in the dark for future use;
[0043] (2) Preparation method of magnetic nanomaterials: Weigh 2.45 g of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com