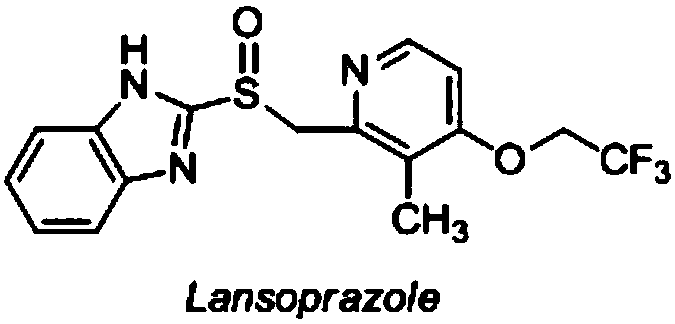
Lansoprazole enteric-coated tablet
A technology of lansoprazole intestines and lansoprazole, which is applied in the field of medicine, can solve problems such as the difference between the onset time and clinical curative effect, increase production costs, and slow down the onset of the drug, and achieve easy operation, good acid resistance, Improve the effect of liver protein synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
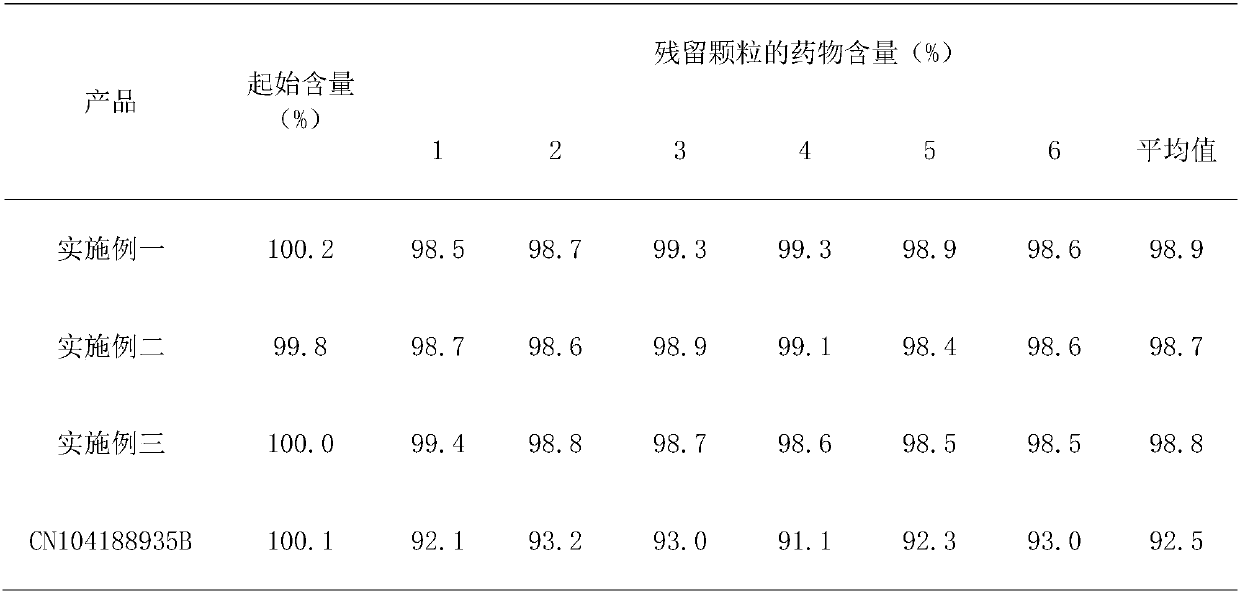
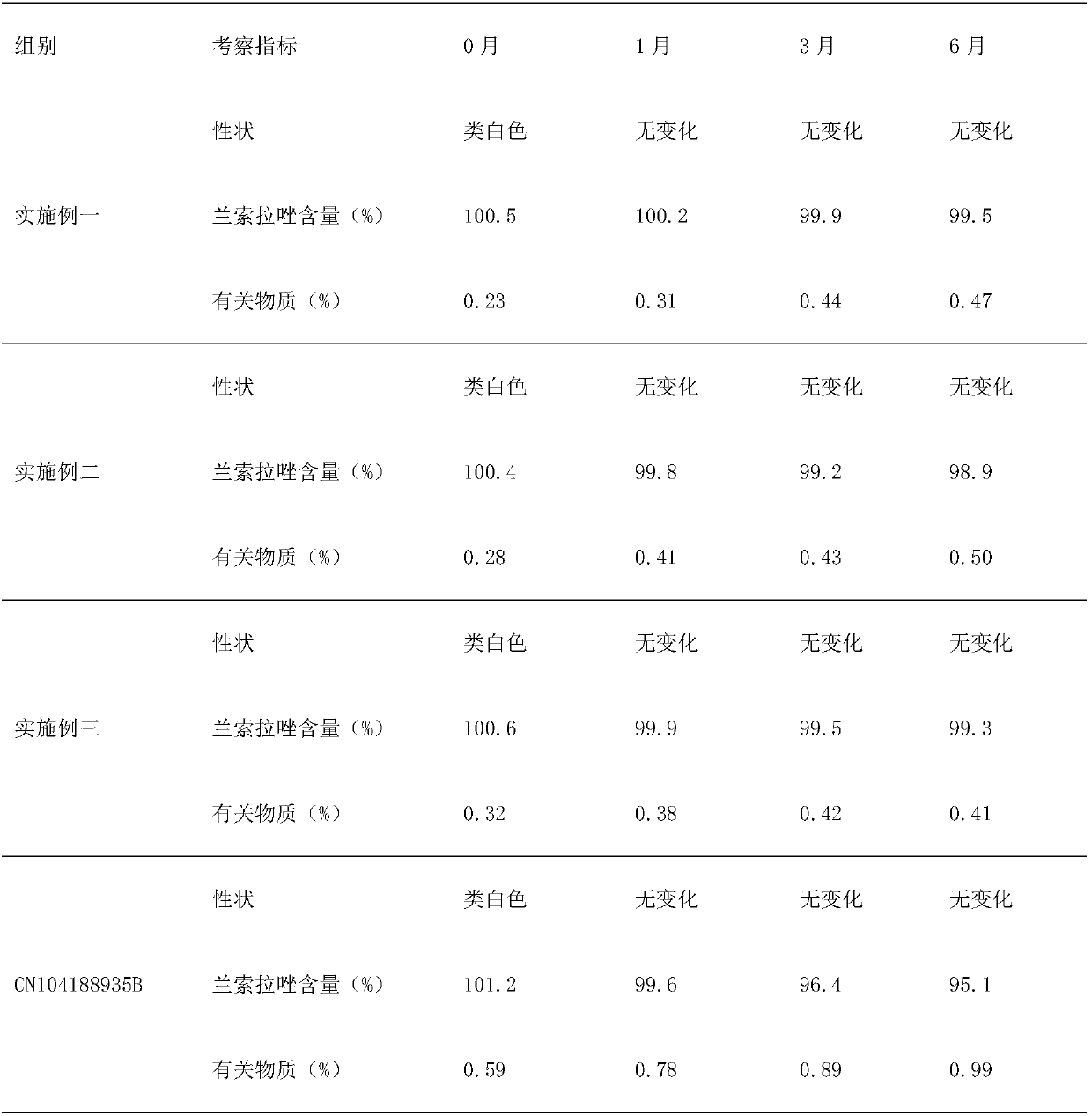
[0015] Embodiment 1: Take lansoprazole, calcium carbonate, hydroxypropyl methylcellulose phthalate, microcrystalline cellulose, alginic acid by weight, count as 1:8:12:3:0.2, grind into fine Powder, mix lansoprazole, calcium carbonate, and microcrystalline cellulose, use alginic acid as a binder, prepare granules, press into tablets, and coat with hydroxypropylmethylcellulose phthalate, that is, Lansoprazole azole enteric-coated tablets.
Embodiment 2
[0016] Embodiment 2: Take lansoprazole, calcium carbonate, hydroxypropyl methylcellulose phthalate, microcrystalline cellulose, alginic acid by weight, count as 1:15:6:6:0.1, grind into fine Powder, mix lansoprazole, calcium carbonate, and microcrystalline cellulose, use alginic acid as a binder, prepare granules, press into tablets, and coat with hydroxypropylmethylcellulose phthalate, that is, Lansoprazole azole enteric-coated tablets.
Embodiment 3
[0017] Embodiment 3: take by weight lansoprazole, calcium carbonate, hydroxypropyl methylcellulose phthalate, microcrystalline cellulose, alginic acid, count as 1:12:8:5:0.15, grind into fine Powder, mix lansoprazole, calcium carbonate, and microcrystalline cellulose, use alginic acid as a binder, prepare granules, press into tablets, and coat with hydroxypropylmethylcellulose phthalate, that is, Lansoprazole azole enteric-coated tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com