Acid-resistant enzyme preparation particles and preparation method thereof
An enzyme preparation and acid-resistant technology, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of increased organic solvent pollution, hidden safety hazards of organic solvents, and high cost of coating materials, etc. problem, to achieve the effect of improving the enzyme activity retention rate, less batch-to-batch variation, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1, a kind of preparation method of acid-resistant phytase
[0026] (1) An acid-resistant phytase granule is provided in this embodiment, and its raw materials include solid raw materials and enteric emulsion, wherein the mass percentage of each component of the solid raw materials: phytase 13%, starch 50%, maltodextrin 17%, talc 20%. Enteric emulsion (30% solid content of methacrylic acid-ethyl acrylate copolymer aqueous solution, the following enteric emulsion refers to this solution) is added at 15% of the solid raw material weight.
[0027] The specific preparation method is as follows:
[0028] Step 1: According to the formula ratio, mix the phytase powder preparation with the rest of the solid raw materials uniformly, first dry-mix for 3 minutes, then add enteric emulsion for wet mixing, and mix for 5 minutes to prepare soft material.
[0029] Step 2: The prepared soft material is shaken or extruded into 20-60 mesh pellets.
[0030] Step 3: Dry the pe...
Embodiment 2
[0047] Embodiment 2, a kind of preparation method of acid-resistant glucose oxidase
[0048] An acid-resistant glucose oxidase granule, the raw materials of the granule include solid raw material and enteric emulsion, wherein the mass percentage of each component of the solid raw material: glucose oxidase 15%, starch 50%, maltodextrin 10%, talc 25% %. The enteric emulsion was added at 15% by weight of the solid raw material.
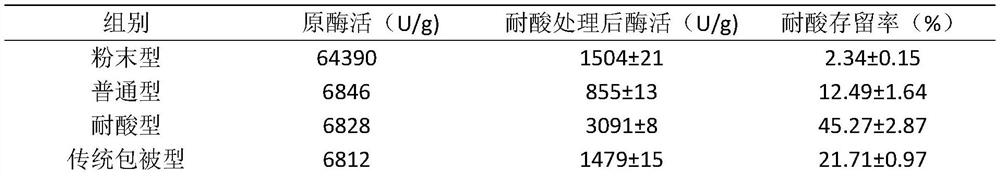
[0049] According to the process of Example 1, acid-resistant glucose oxidase granules, powder-type glucose oxidase granules, and ordinary glucose oxidase granules were prepared respectively for acid resistance evaluation, and the enzyme activity retention rate was calculated respectively. The specific results are shown in Table 2.
[0050] Table 2 Effects of glucose oxidase granules prepared by different methods on acid resistance
[0051]
[0052] It can be seen from the results in Table 2 that the enzyme activity retention rate of acid-resistant ...
Embodiment 3
[0053] Embodiment 3, the influence of the addition amount of enteric emulsion on acid resistance
[0054] According to the preparation method of acid-resistant glucose oxidase enzyme granules in Example 1, the addition amount of the enteric emulsion was screened, and the enzyme activity retention rate was used as the evaluation index. The results are shown in Table 3.
[0055] Table 3 Effects of different enteric emulsion additions on acid resistance and enzyme activity retention rate of glucose oxidase granules
[0056]
[0057]
[0058] It can be seen from the results in Table 3 that the addition amount of enteric emulsion is negatively correlated with the original enzyme activity of glucose oxidase granules, and the addition amount of enteric emulsion is positively correlated with the enzyme activity retention rate. Considering the application effect and cost, the recommended addition amount is 5-30%, more preferably 5-20%, and the preferred addition amount is 15%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com