Diethylstibestrol hapten and complete antigen and preparation method thereof
A complete antigen and hapten technology, which is applied in the field of immunochemistry and detection, can solve the problems of low product purity, uncontrollable reaction, and long reaction time, and achieve the effects of high product purity, saving reaction substrates, and short synthesis time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
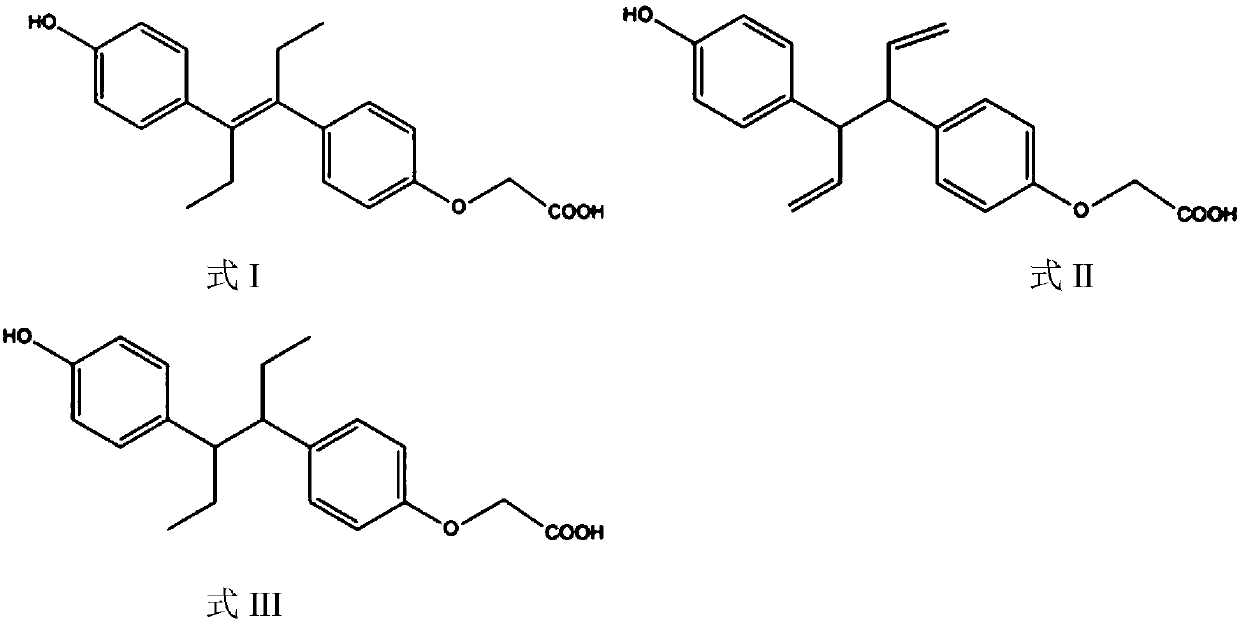
[0018] The estrogen hapten is an estrogen derivative, and the hapten is the introduction of a carboxyl group on the phenolic hydroxyl side of estrol to form an estrol-monocarboxymethyl ether compound.
[0019] Specifically, the structural formulas of the estrogen haptens prepared in the examples of the present invention are shown in Formula I, Formula II, and Formula III:
[0020]
[0021] As described in the background art, the existing synthesis of estrogen haptens has many shortcomings. The present invention redesigns and optimizes the hapten synthesis process, and finally obtains an efficient synthesis method of high-purity estrogen haptens.
[0022] In a specific embodiment, the present invention discloses a method for preparing estrogen haptens, the method comprising: carrying out estrogen and monochloroacetate in an organic solvent at room temperature and the presence of a catalyst The reaction is terminated by ice water, the organic extractant extracts unreacted estrogens, th...
Embodiment 1
[0036] Example 1: Preparation method of diethylstilbestrol hapten
[0037] Weigh 295 mg of diethylstilbestrol into 6 mL of dry dimethyl sulfoxide, add 1.540 g of potassium hydroxide to the solution, and stir for 5 min. Add 64 mg of sodium monochloroacetate. At room temperature 10°C, after 35 minutes of magnetic stirring, 50 mL of ice water was added to terminate the reaction. Add 24 mL of ethyl acetate and extract three times to remove unreacted diethylstilbestrol. Under stirring, the aqueous phase was acidified with 100 mL 1.5mol / L hydrochloric acid, and a white precipitate appeared. Filter through a funnel, wash the precipitate with distilled water to neutrality, and place it in an oven to dry to obtain the diethylstilbestrol hapten with a yield of about 28% and a purity of about 98%.
Embodiment 2
[0038] Example 2: Preparation method of diethylstilbestrol hapten
[0039] Weigh 293 mg of diethylstilbestrol into 4 mL of dry dioxane, add 0.924 g of potassium hydroxide to the solution, and stir for 5 min. Add 128 mg of sodium monochloroacetate. At room temperature 10°C, after 35 minutes of magnetic stirring, 50 mL of ice water was added to terminate the reaction. Add 24 mL of ethyl acetate and extract twice to remove unreacted diethylstilbestrol. The aqueous phase was acidified with 100mL 2.5mol / L nitric acid while stirring, and a white precipitate appeared. Filter through a funnel, wash the precipitate with distilled water until it is neutral, put it in an oven to dry, and obtain the diethylstilbestrol hapten with a yield of about 29% and a purity of about 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com