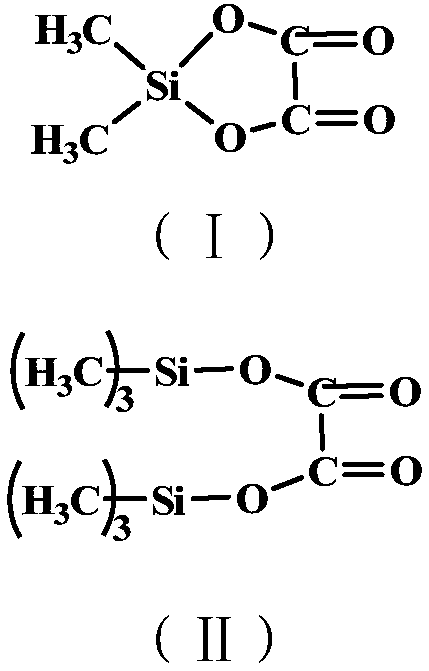Method for synthesizing an electrolyte lithium salt: lithium difluoro(oxalate)borate
A technology of lithium difluorooxalate borate and synthesis method, which is applied in chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, organic chemistry, etc., and can solve the problem of low oxidation potential, poor thermal stability, and influence on electrolyte Performance and other issues, to achieve the effect of high reaction conversion rate and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the synthesis of lithium difluorooxalate borate (LiODFB)
[0037] (1) Synthesis of condensate of silane oxalic acid
[0038] In a flask equipped with a magnetic stirrer, a thermometer, a reflux condenser and a nitrogen conduit, add 50 g of oxalic acid, stir and raise the temperature to 80°C, then slowly add 71.7 g of dimethyldichlorosilane, and the reaction ends after 24 hours. The reaction system was distilled off the solvent under reduced pressure at 120° C. to obtain 80.1 g of a colorless viscous product, which was a condensate of silane oxalic acid. The obtained product was analyzed, and the result showed that the purity was 99.3% (calculated by nuclear magnetic spectrum).
[0039] (2) Synthesis of lithium difluorooxalate borate (LiODFB)
[0040] Weigh 20g lithium tetrafluoroborate, 32.7g condensate of silane oxalic acid in the above-mentioned step (1), 150g dimethyl carbonate in the glove box, join in the three-necked flask that magnetic stirring, t...
Embodiment 2
[0042] Embodiment 2: the synthesis of lithium difluorooxalate borate (LiODFB)
[0043] (1) Synthesis of condensate of silane oxalic acid
[0044] In a flask equipped with a magnetic stirrer, a thermometer, a reflux condenser and a nitrogen conduit, add 50 g of oxalic acid, stir and raise the temperature to 50 ° C, then slowly add 52.5 g of dimethyl monochlorosilane, and the reaction ends after 24 hours. The reaction system was distilled off the solvent under reduced pressure at 120° C. to obtain 78.6 g of a colorless viscous product, which was a condensate of silane oxalic acid. The obtained product was analyzed, and the result showed that the purity was 99.1% (calculated by nuclear magnetic spectrum).
[0045] (2) Synthesis of lithium difluorooxalate borate (LiODFB)
[0046] In the glove box, take by weighing 30g lithium tetrafluoroborate, the condensate of silane oxalic acid in the above step (1) of 49.1g, the diethyl carbonate of 240g, join in the three-necked flask that ...
Embodiment 3
[0048] Embodiment 3: the synthesis of lithium difluorooxalate borate (LiODFB)
[0049] (1) Synthesis of condensate of silane oxalic acid
[0050] In a flask equipped with a magnetic stirrer, a thermometer, a reflux condenser and a nitrogen conduit, add 50g of oxalic acid, stir and raise the temperature to 60°C, then slowly add 120.7g of trimethylchlorosilane, and the reaction ends after 30h. The reaction system was distilled off the solvent under reduced pressure at 120° C. to obtain 123.5 g of a colorless viscous product, which was a condensate of silane oxalic acid. The obtained product was analyzed, and the result showed that the purity was 98.7% (calculated by nuclear magnetic spectrum).
[0051] (2) Synthesis of lithium difluorooxalate borate (LiODFB)
[0052] Weigh 20g lithium tetrafluoroborate, 51.6g condensate of silane oxalic acid in the above-mentioned step (1), 150g diethyl carbonate in the glove box, join in the three-necked flask that magnetic stirring, thermome...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com