Synthesis method of copolyester
A synthesis method and copolyester technology, applied in the field of copolyester synthesis, can solve the problems of limited types of monomers, difficulty in obtaining high molecular weight polymers, rare copolymerization reactions, etc. The effect of a wide temperature range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
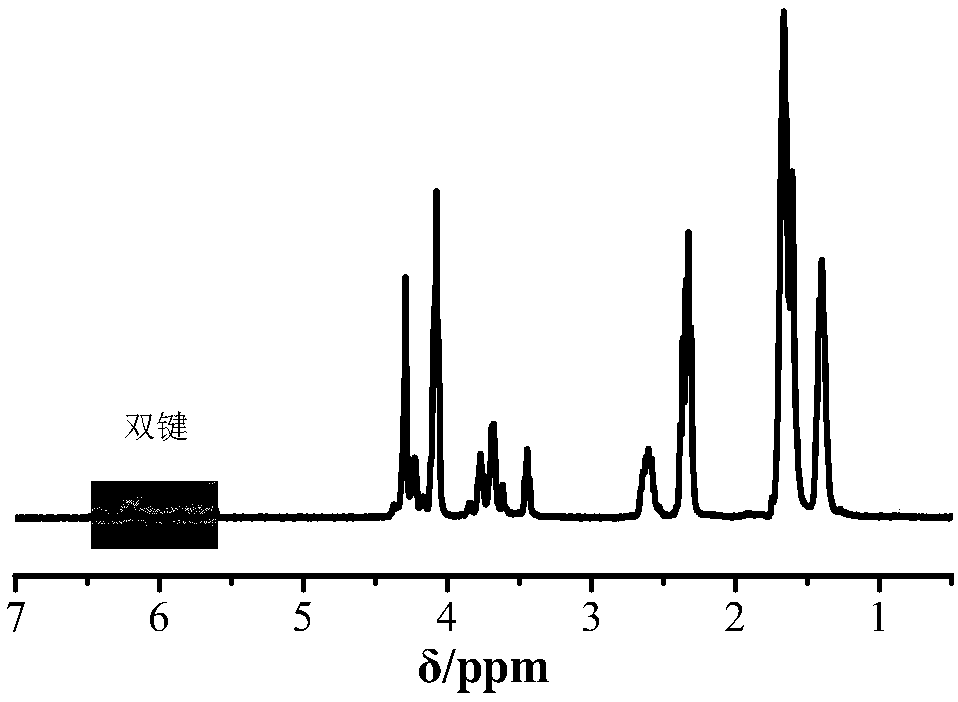
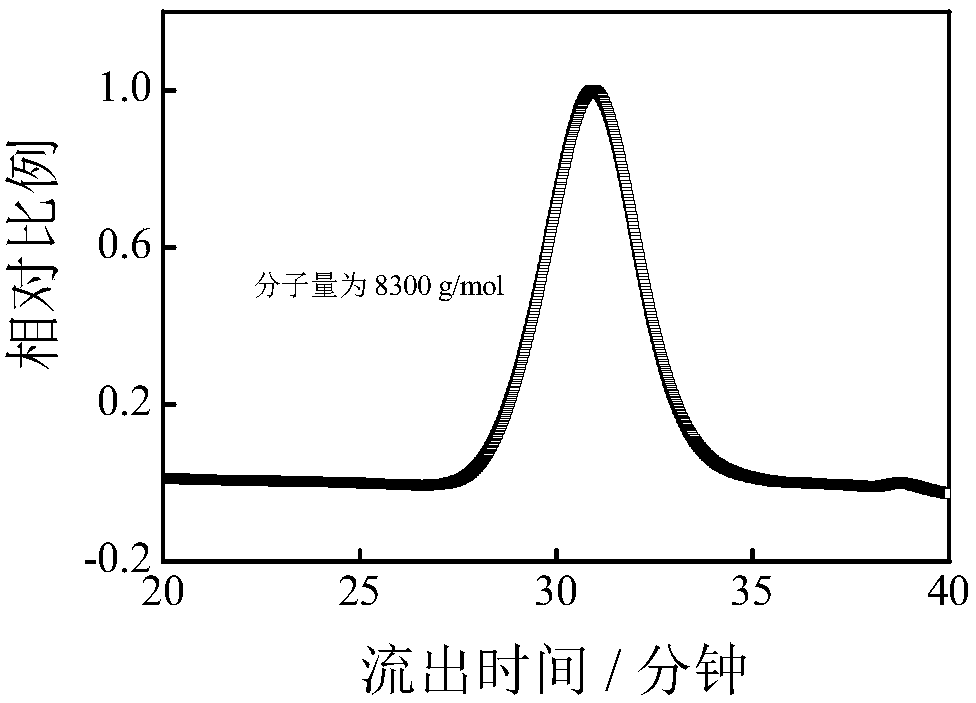
[0022] Take 0.2g (1.7mmol) of allyl hydroxyethyl ester and 0.8g (7.0mmol) of caprolactone into a 10mL round bottom flask with a rotor, then add 1g of tetrahydrofuran as a solvent, freeze with liquid nitrogen, and replace the argon three times After that, add 43.8 μl (0.09 mmol) phosphazene base P 2 . The reaction was placed in a 60°C oil bath for 0.5h. After the reaction, the reaction product was dissolved with tetrahydrofuran, terminated with methanol, and then precipitated in 50mL of n-hexane to obtain a white product, which was vacuum-dried at 30°C for 12 hours, wherein the conversion rate of hydroxyethyl acrylate reached 97.5%, and the conversion rate of caprolactone Up to 99.8%. Its number average molecular weight is 8300g / mol, molecular weight distribution is 1.73, see figure 2 The proton nuclear magnetic spectrum and gel permeation chromatogram of the obtained product, it can be seen that the polymer is not subjected to traditional vinyl polymerization, but is carri...
Embodiment 2
[0024] Take 0.2g (1.7mmol) of allyl hydroxyethyl ester and 0.8g (7.0mmol) of caprolactone into a 10mL round bottom flask with a rotor, then add 1g of tetrahydrofuran as a solvent, freeze with liquid nitrogen, and replace the argon three times After that, add 43.8 μl (0.09 mmol) phosphazene base P 2 . The reaction was placed in a 60°C oil bath for 12 hours. After the reaction, the reaction product was dissolved in tetrahydrofuran, terminated with methanol, and then precipitated in 50 mL of n-hexane to obtain a white product, which was vacuum-dried at 30°C for 12 hours, wherein the conversion rate of hydroxyethyl acrylate reached 98.5%, and the conversion rate of caprolactone Up to 99.9%. Its number-average molecular weight is 8500g / mol, and its molecular weight distribution is 1.79. According to the hydrogen nuclear magnetic spectrum and gel permeation chromatogram of the obtained product, it can be seen that the polymer has not undergone traditional vinyl polymerization, but...
Embodiment 3
[0026] Take 0.2g (1.7mmol) of allyl hydroxyethyl ester and 0.8g (7.0mmol) of caprolactone into a 10mL round bottom flask with a rotor, then add 1g of tetrahydrofuran as a solvent, freeze with liquid nitrogen, and replace the argon three times After that, add 43.8 μl (0.09 mmol) phosphazene base P 2 . The reaction was placed in a low-temperature constant temperature bath at -20°C, and reacted for 1 h. After the reaction, the reaction product was dissolved in tetrahydrofuran, terminated with methanol, and then precipitated in 50 mL of n-hexane to obtain a white product, which was vacuum-dried at 30°C for 12 hours, wherein the conversion rate of hydroxyethyl acrylate reached 82.3%, and the conversion rate of caprolactone up to 88.2%. Its number-average molecular weight is 7100g / mol, and its molecular weight distribution is 1.67. According to the hydrogen nuclear magnetic spectrum and gel permeation chromatogram of the obtained product, it can be seen that the polymer has not un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com