Organic compound based on gallocyanine and application thereof
An organic compound and catalytic reaction technology, applied in the field of fluorescent probes, can solve the problems that the external environment cannot simultaneously detect reactive oxygen species, reactive oxygen species cells, and biological damage cannot be fed back in time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of organic compound of formula I based on cyanine:
[0028] (1) Preparation of Compound 1
[0029] Under the protection of argon, (4-bromobutyl)triphenylphosphine bromide (14.35g, 30mmol) and sodium azide (3.9g, 60mmol) were dissolved in 50mL DMF. Stir overnight at 90°C. The color of the solution ranges from colorless to light yellow to red. The reaction flask was cooled to room temperature, and 50 mL of dichloromethane was added until a large amount of precipitation occurred. Filter and collect the filtrate. The collected filtrate was extracted with ethyl acetate and water 1:1 (v / v), the organic phase was collected, and rotary evaporated. Put the product after rotary evaporation into a round-bottomed flask, install a reflux device, stir, add about 7.5 ml of dichloromethane to dissolve it completely, heat the solution to a temperature of about 40°C, and reflux. When the solution boiled slightly, ethyl acetate was added to make the solution white crystal...
Embodiment 2
[0051] The prepared compound of formula I was used as a probe in cells, tissues and organs to detect MAO-B and the reactive oxygen species produced by its catalysis, simulating physiological conditions, and the following experiments were all carried out under the condition of pH=7.4 (HEPES buffer solution, the concentration is 40 mM), and the concentration of the probe is 10 μM.
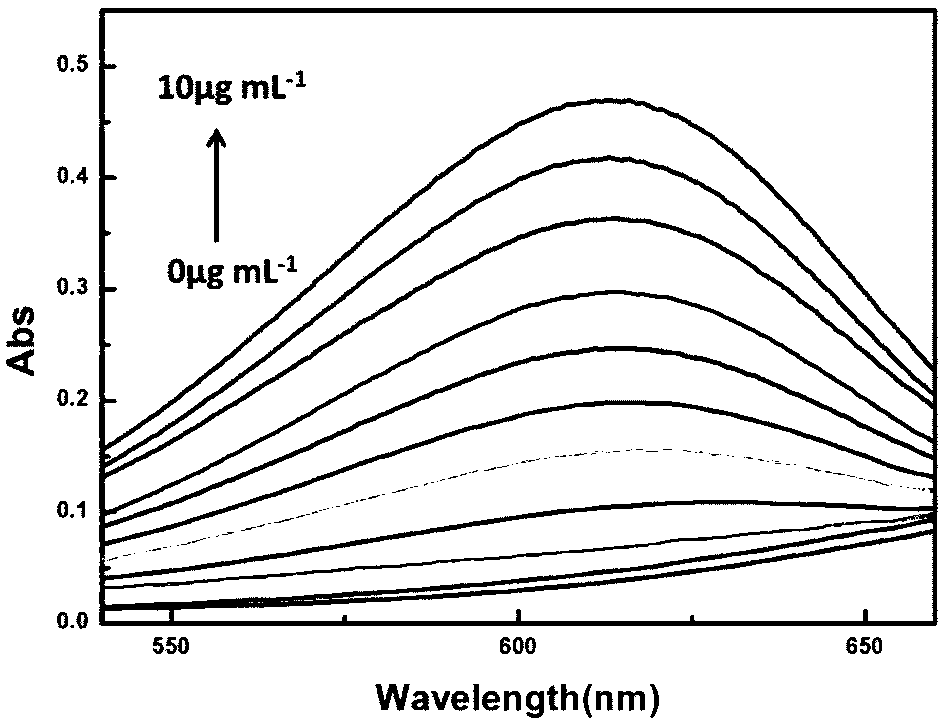
[0052] The ultraviolet response of the compound of formula I prepared above to MAO-B and the active oxygen species produced by its catalysis:
[0053]pH was controlled with HEPES buffer solution. Add the compound of formula I to a 10mL colorimetric tube to ensure its final concentration is 10μM, then add 40mM HEPES, then add MAO-B, add 10mL of ultrapure water to make the concentration of MAO-B respectively 0μg mL -1 , 1 μg mL -1 , 2 μg mL -1 , 3 μg mL -1 , 4 μg mL -1 , 5 μg mL -1 , 6 μg mL -1 , 7 μg mL -1 , 8 μg mL -1 , 9 μg mL -1 , 10 μg mL -1 . Shake the solution and equilibrate for 60 ...
Embodiment 3
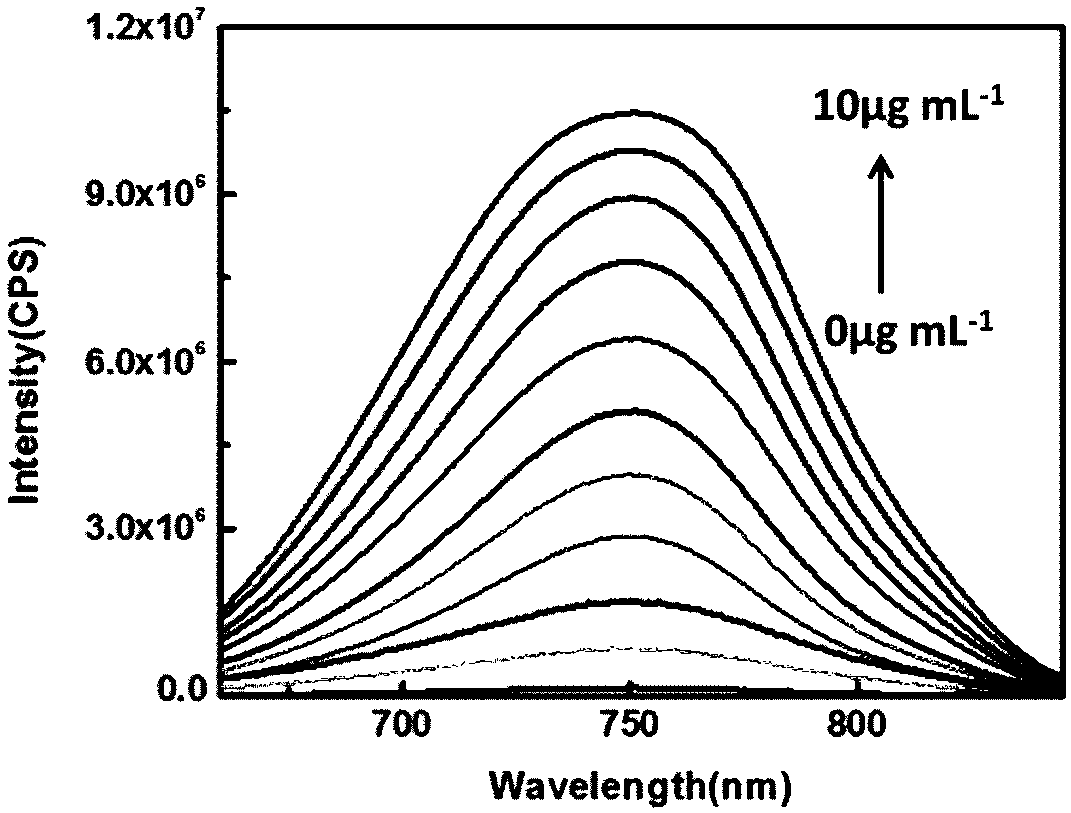
[0056] The fluorescence response of the compound of formula I to MAO-B and the reactive oxygen species produced by its catalysis:
[0057] pH was controlled with HEPES buffer solution. Add the compound of formula I to a 10mL colorimetric tube to ensure its final concentration is 10μM, then add 40mM HEPES, then add MAO-B, add 10mL of ultrapure water to make the concentration of MAO-B respectively 0μg mL -1 , 1 μg mL -1 , 2 μg mL -1 , 3 μg mL -1 , 4 μg mL -1 , 5 μg mL -1 , 6 μg mL -1 , 7 μg mL -1 , 8 μg mL -1 , 9 μg mL -1 , 10 μg mL -1 . Shake the solution evenly and equilibrate for 60 minutes, then add the above working solution into the fluorescence dish to measure the fluorescence spectrum. The changes of fluorescence spectrum before and after detection of MAO-B are as follows: figure 2 shown. The compound of formula I can be used to realize the detection of MAO-B and the active oxygen species produced by its catalysis in organisms.
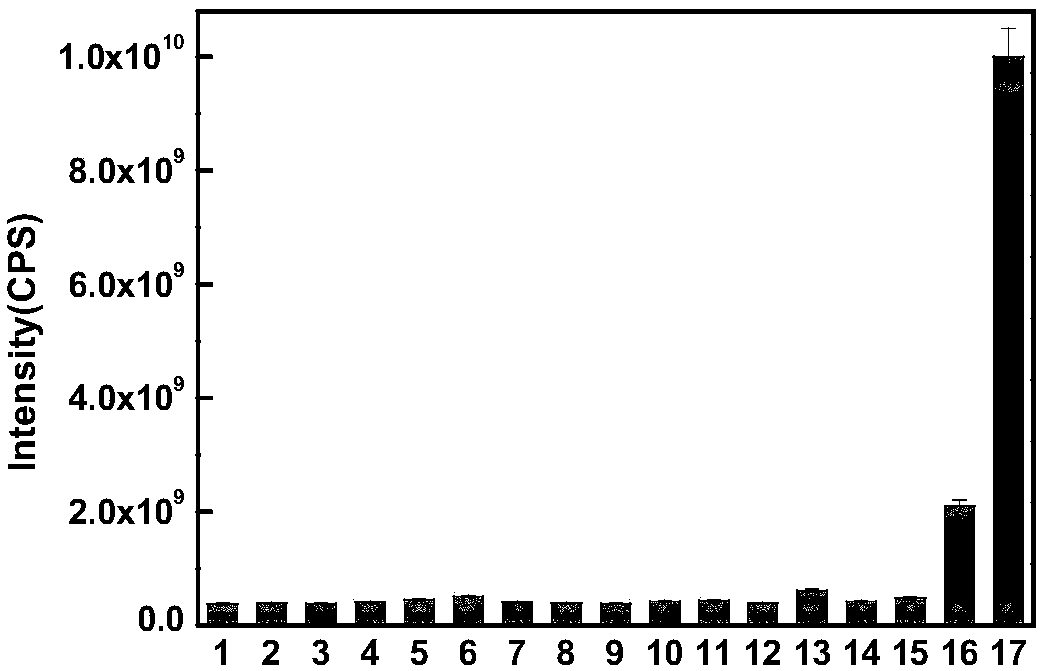
[0058] Depend on figure ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com