Fluorenyl oxadiazole iridium complexes, and preparation method and application thereof
A technology of fluorenyl oxadiazoles and iridium complexes, which is applied in the direction of indium organic compounds, platinum group organic compounds, chemical instruments and methods, etc., which can solve the problems of device efficiency reduction, etc., to weaken stacking and increase steric hindrance , The effect of low raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
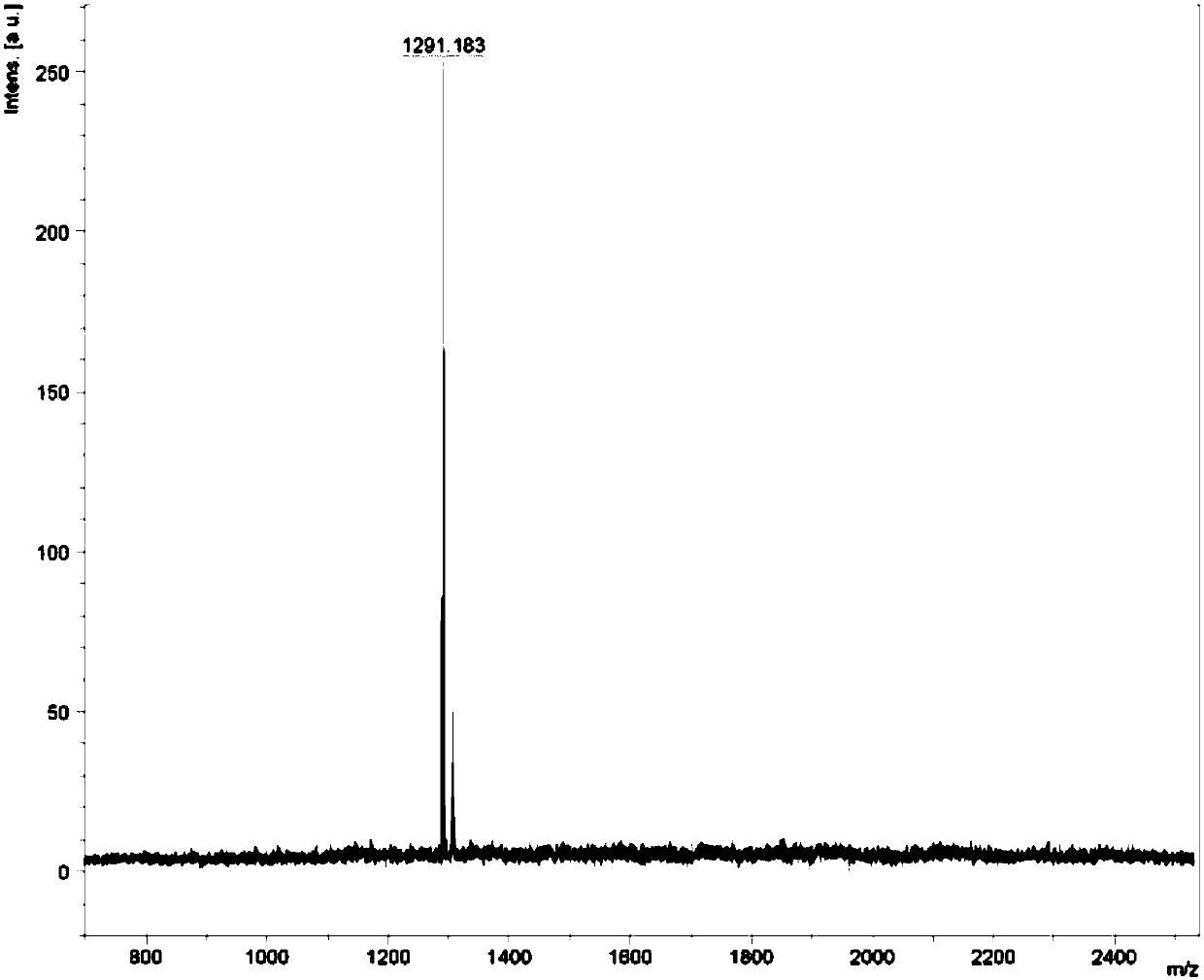
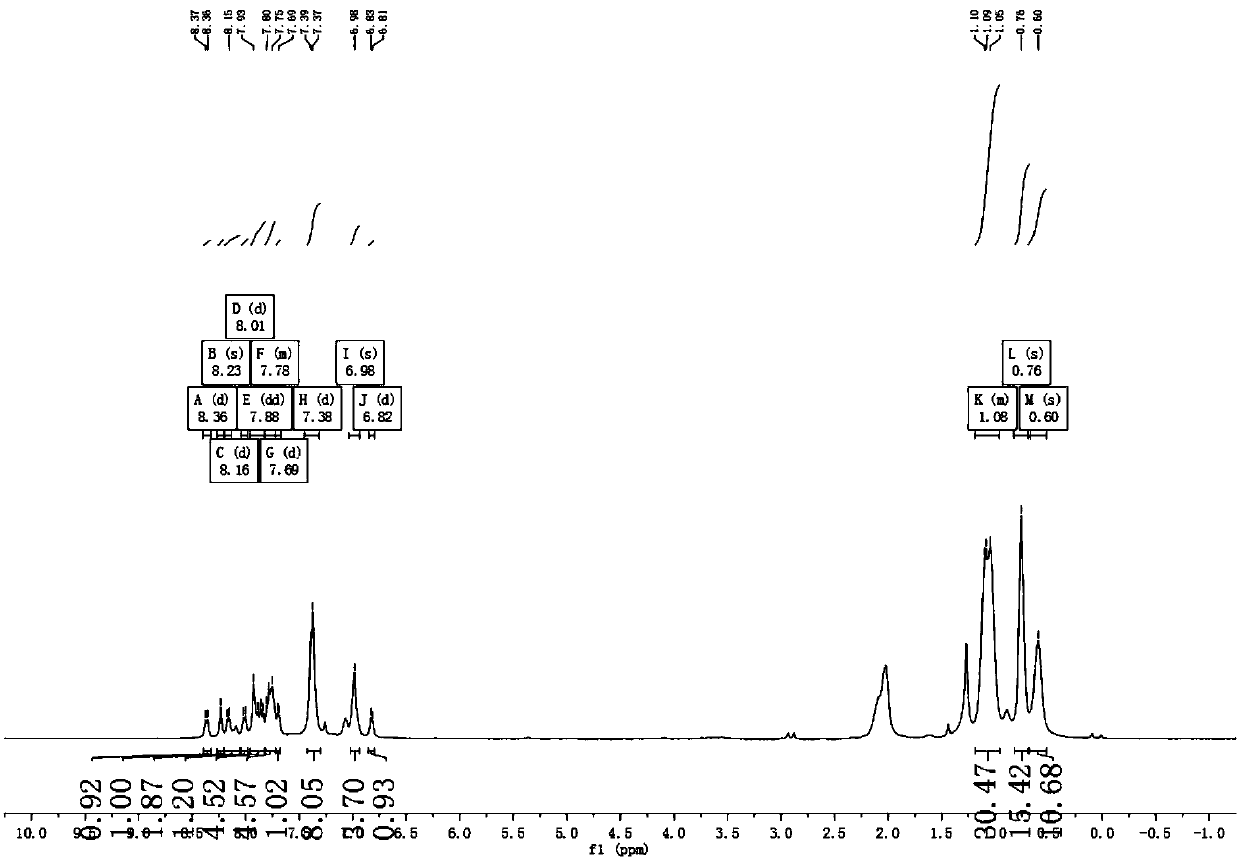
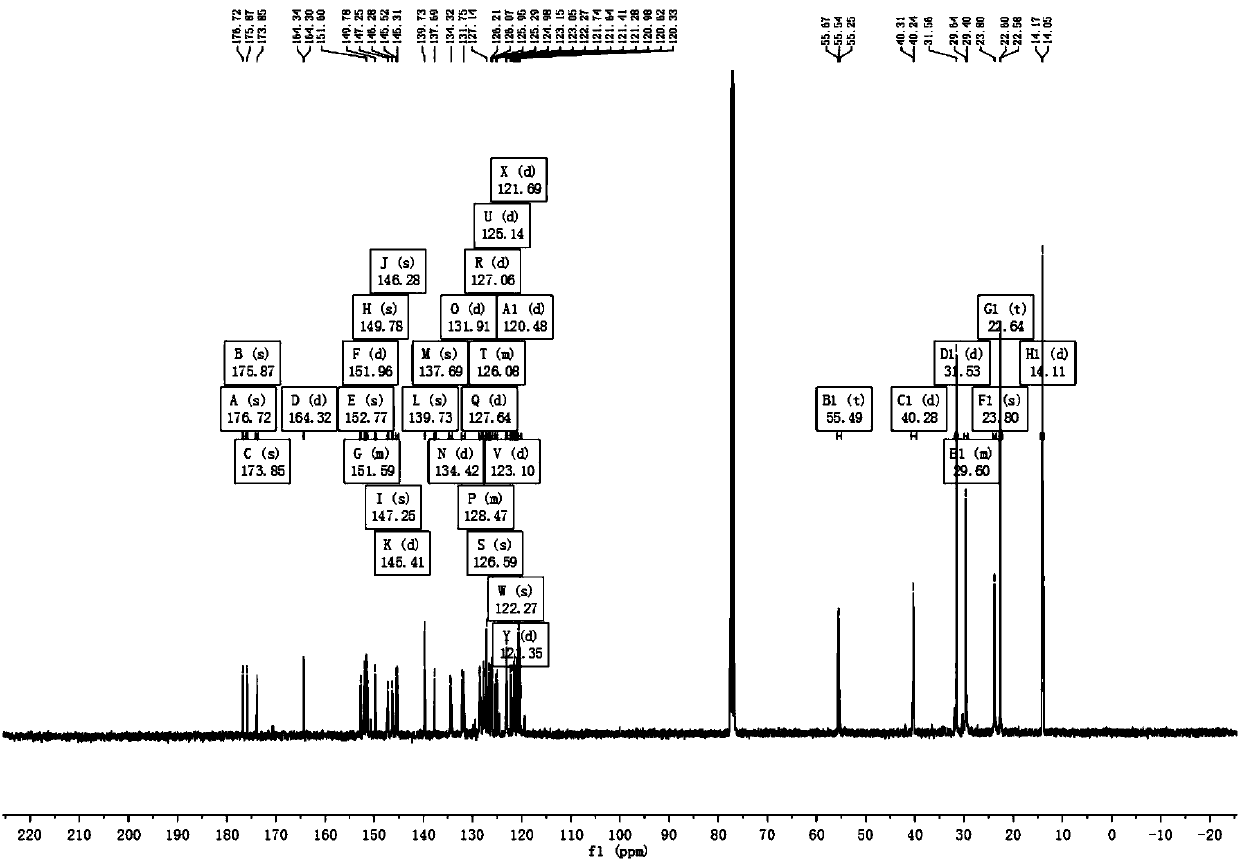
[0055] Example 1 Complex Ir(HFFB) 2 Synthesis of (pic)
[0056] The synthetic route is as follows:
[0057]
[0058] The specific implementation method is as follows:
[0059] Step 1, add compound 1 (20g, 80mmol), tetrabutylammonium bromide (1.315g, 4.1mmol), 50% NaOH 8mL, DMSO 50mL to a 250mL three-necked flask, stir at room temperature for half an hour, then add hexyl bromide (29.618g, 179.5mmol), turn on the stirrer and raise the temperature to 45°C, and react for 8h. After the reaction, it was extracted several times with petroleum ether and deionized water, the upper organic layer was dried with anhydrous magnesium sulfate, and purified by column chromatography (eluent: ethyl acetate: petroleum ether = 1:20). A transparent oily liquid was obtained, and compound 2, namely 2-bromo-9,9-dihexylfluorene (30.1 g, yield 89%) was obtained after vacuum drying.
[0060] Step 2, add compound 2 (30.1g, 72.9mmol) and cuprous cyanide (13.1g, 115.8mmol) into a 250mL three-neck fl...
Embodiment 2
[0068] The synthesis of embodiment 2 complex compound Ir (HFFF) 2 (pic)
[0069] The synthetic route is shown in Example 1.
[0070] The specific implementation method is as follows:
[0071] Step 1, add compound 1 (5g, 20mmol), tetrabutylammonium bromide (0.329g, 1.02mmol), 50% NaOH 2mL, DMSO 15mL into a 100mL three-necked flask, stir at room temperature for half an hour, then add hexyl bromide (7.404g, 44.87mmol), turn on the stirrer and raise the temperature to 45°C, and react for 8h. After the reaction, it was extracted several times with petroleum ether and deionized water, the upper organic layer was dried with anhydrous magnesium sulfate, and purified by column chromatography (eluent: ethyl acetate: petroleum ether = 1:20). A transparent oily liquid was obtained, and compound 2, 2-bromo-9,9-dihexylfluorene (7.5 g, yield 89%) was obtained after vacuum drying.
[0072] Step 2, add compound 2 (7.5g, 18.22mmol) and cuprous cyanide (3.275g, 28.95mmol) into a 100mL three-n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com