Traditional Chinese medicine composition for preventing and treating mammary gland hyperplasia and preparation method thereof
A technology of mammary gland hyperplasia and composition, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations containing active ingredients, etc., which can solve problems such as difficult to deal with complex and changeable, low pertinence of patented medicines, and complex and changeable mammary gland hyperplasia syndromes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
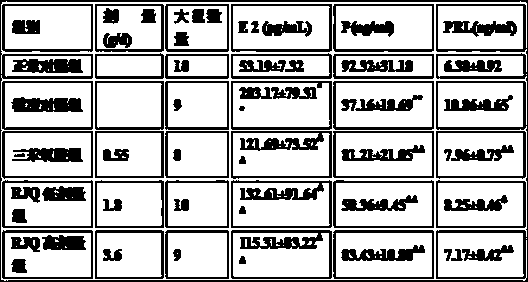
[0055] [Experimental Example 1] Therapeutic effect of the medicine of the present invention on hormone-induced mammary enlargement in rats
[0056] 1 material
[0057] 1.1 Animals
[0058] SD healthy non-pregnant rats, female, weighing 180-220 g, were provided by the Experimental Animal Center of Hebei Medical University, and were adaptively fed for one week before the experiment.
[0059] Reagents and Drugs
[0060] Estradiol benzoate (1mL: 2mg × 10 sticks) and progesterone (1mL: 10mg × 10 sticks) injections were purchased from Tianjin Jinyao Pharmaceutical Co., Ltd. (batch numbers 150215 and 150312 respectively), E 2 , P, and PRL radioimmunoassay kits were purchased from Beijing Furui Bioengineering Company (batch number 150603), and tamoxifen was produced by Yangzijiang Pharmaceutical Group Co., Ltd. (batch number 15041501).
[0061] Rujieqing (RJQ) prescription Chinese medicine decoction pieces were purchased from Lerentang Pharmaceutical Group Co., Ltd., and were ident...
experiment example 2
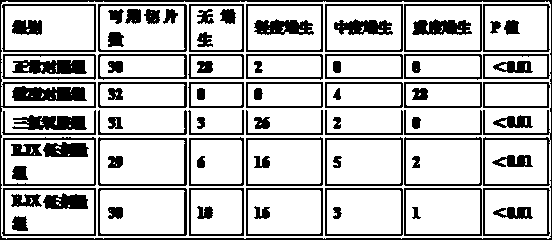
[0097] [Experimental example 2] the clinical observation of drug treatment mammary gland hyperplasia of the present invention
[0098] 1 Case information:
[0099] 130 outpatients were selected; all were female patients; 31 patients aged 20-30 years, 60 patients aged 30-40, 29 patients aged 40-50, 10 patients aged 50-60, 130 patients were randomly divided into treatment group, Control group; 65 people in the treatment group (average age 34 years old, disease course 3 months to 6 years, 32 people with unilateral breast disease, 33 people with bilateral breast disease); 65 people in the control group (average age 33 years old, disease course 5 months ——8 years, 30 patients with unilateral breast disease and 35 patients with bilateral breast disease); the data of the two groups are comparable in terms of age, disease duration, and disease distribution.
[0100] Diagnostic criteria
[0101] Refer to the diagnostic criteria of mammary gland hyperplasia in "Surgery": unilateral or...
Embodiment 1
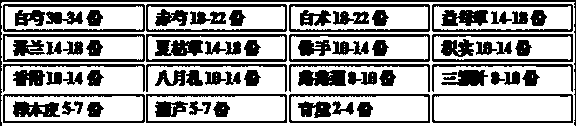
[0118] [embodiment 1] medicine capsule of the present invention
[0119] a) Weigh the raw drug for later use, the dosage of the raw drug is as follows:
[0120] 32 parts of white peony
20 parts of red peony
Atractylodes 20 parts
Motherwort 16 parts
Zeeland 16 servings
Prunella vulgaris 16 parts
bergamot 12 servings
Citrus aurantium 12 parts
Cyperus 12 parts
August letter 12 copies
Passepartout 9 copies
9 copies of three needles
6 parts birch bark
6 servings of oatmeal
Indigo Daisy 3 parts
[0121] b) decocting the 6 traditional Chinese medicines of Radix Paeoniae Rubra, Atractylodes Rhizoma Atractylodes Rhizome, Atractylodes Rhizoma Atractylodes Rhizoma Atractylodes Rhizoma Atractylodes Rhizoma Atractylodes Rhizoma Atractylodes Rhizoma Atractylodes Rhizoma Atractylodes Rhizoma Atractylodes Rhizoma Atractylodes Rhizoma Atractylodes Rhizoma Atractylodes Rhizoma Atractylodes Rhizoma Atractylodes Rhi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com