Total-biology-base benzoxazine resin and preparation method thereof
An oxazine resin and all-biological technology, which is applied in the field of thermosetting resins, can solve the problems of poor heat resistance of benzoxazines, synthetic routes and methods to be explored, and insufficient heat resistance, so that no inert gas protection is required, Effect of good substrate adhesion and surface hydrophobicity, good corrosion protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
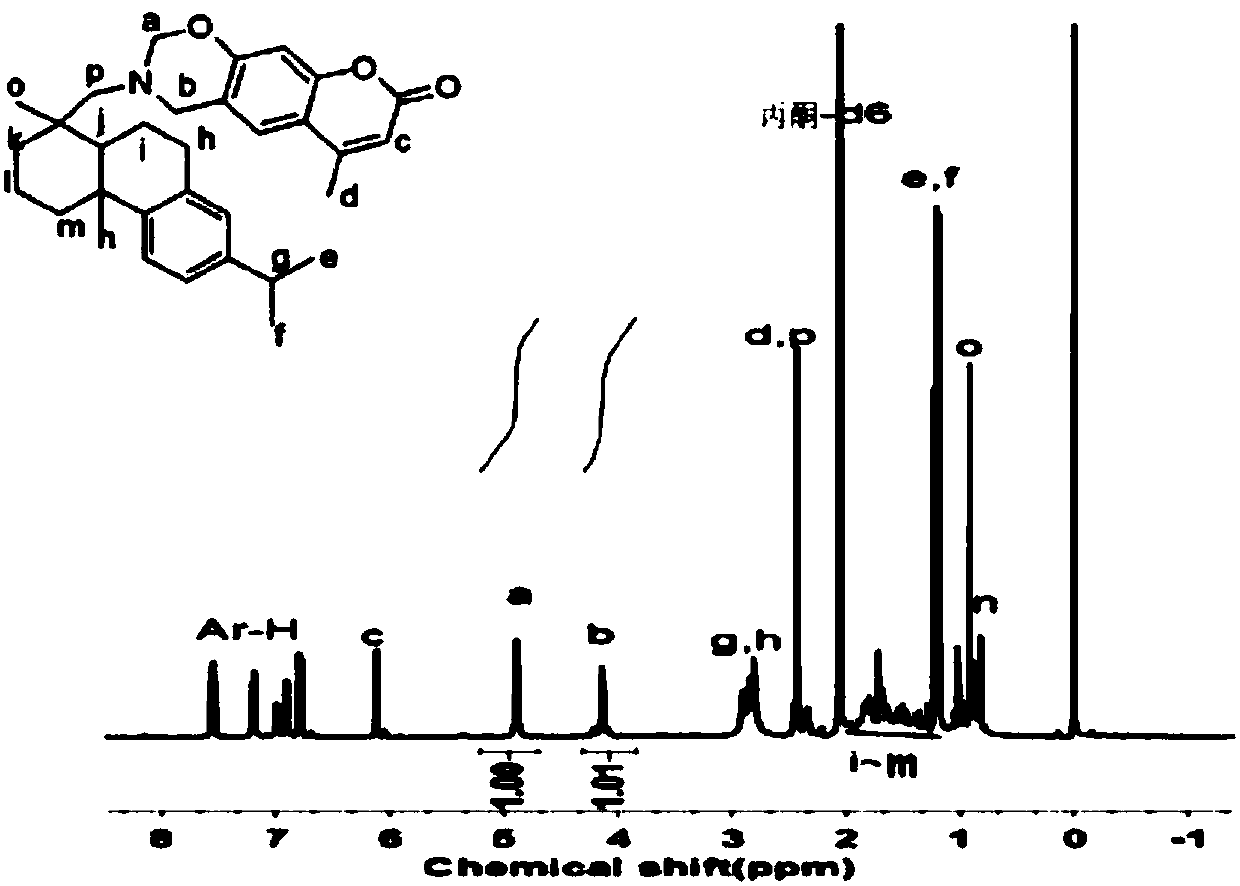
[0039] (1) In a three-necked flask equipped with magnetic stirring and condensing reflux device, add 0.5mol of dehydroabietamine, 0.5mol of 7-hydroxy-4-methylcoumarin, 1mol of paraformaldehyde and solvent dioxane, Heat to 70°C, wait until the mixture is completely dissolved, then raise the temperature to 85°C, and react for 20 hours. The system is a transparent yellow-brown liquid. After cooling to room temperature, most of the dioxane solvent is removed by rotary evaporation at 60°C, then washed three times with 1% sodium bicarbonate solution, and finally washed with a large amount of hot deionized water to medium properties, a light yellow crude product can be obtained. The crude product was crystallized with ethanol, filtered and dried to obtain a pure bio-based benzoxazine monomer containing a phenanthrene ring structure.
[0040] The structure of the all bio-based benzoxazine monomer containing the phenanthrene ring structure in this embodiment is:
[0041]
[0042] ...
Embodiment 2
[0050] The 7-hydroxyl-4-methylcoumarin in embodiment 1 is changed into guaiacol, adds dehydroabietamine 1mol, guaiacol 1.1mol, paraformaldehyde 2.1mol and solvent dioxane, Heat to 75°C, wait until the mixture is completely dissolved, then raise the temperature to 88°C, and react for 22 hours. Other steps are the same as in Example 1.
[0051] The structure of the all bio-based benzoxazine monomer containing the phenanthrene ring structure in this embodiment is:
[0052]
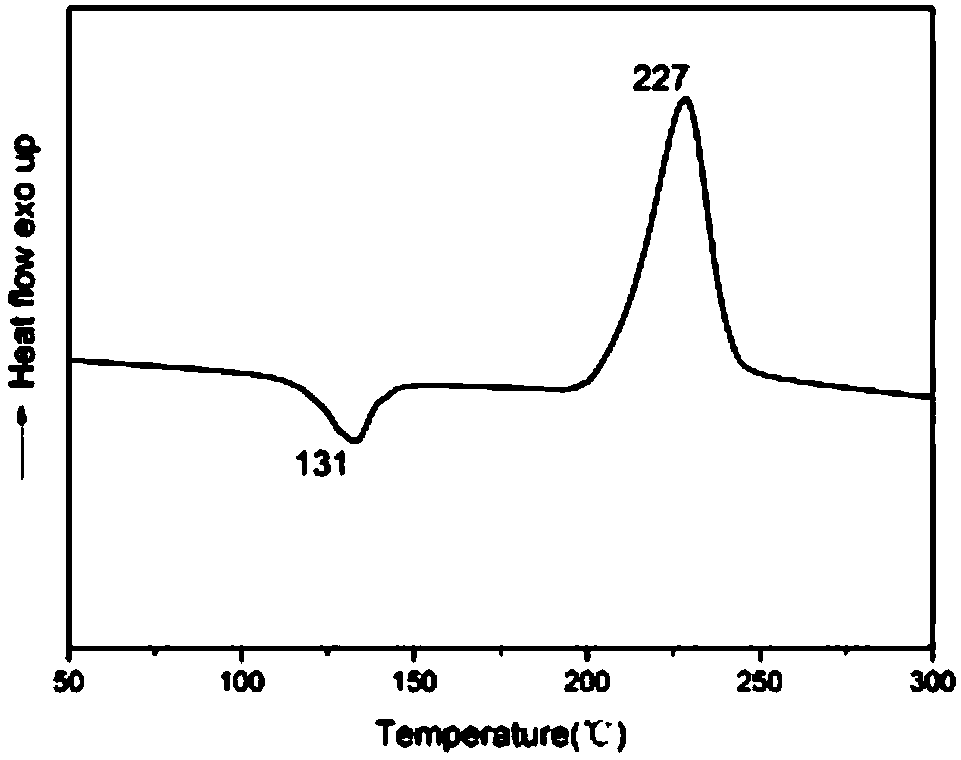
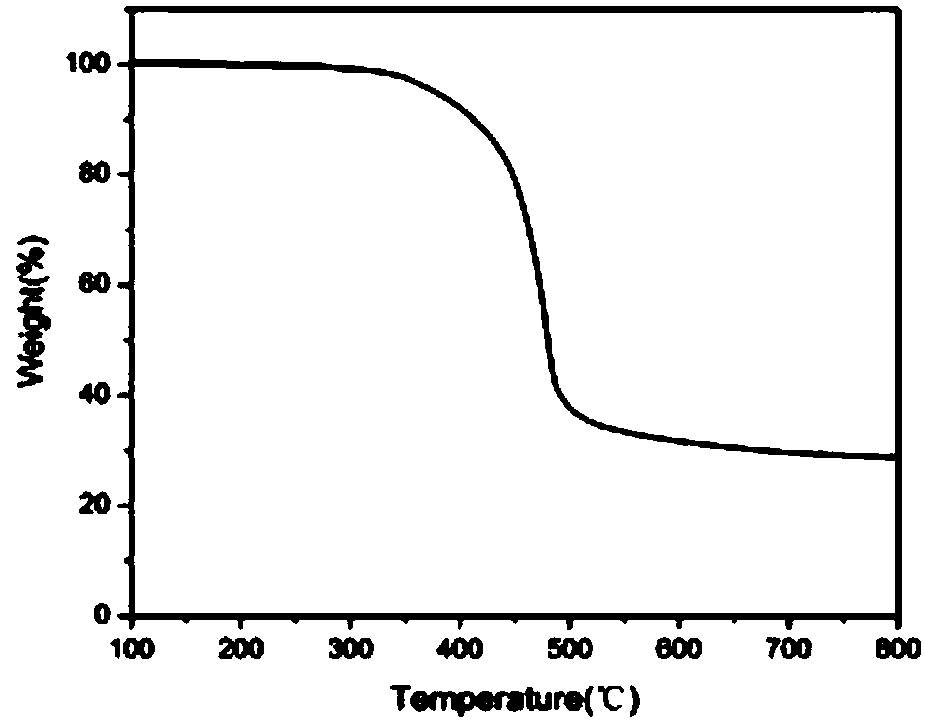
[0053] The initial and maximum ring-opening temperatures of the all-bio-based benzoxazine containing the phenanthrene ring structure in this example are 180°C and 225°C respectively, the residual carbon rate is 24%, and T 5 =321°C, T 10 = 358°C.
Embodiment 3
[0055] The phenolic source in embodiment 2 is changed into vanillin. Other operating steps are the same as those in Example 2.
[0056] The structure of the all bio-based benzoxazine monomer containing the phenanthrene ring structure in this embodiment is:
[0057]
[0058] The initial and maximum ring-opening temperatures of the all-bio-based benzoxazine containing the phenanthrene ring structure in this example are 180°C and 225°C respectively, the residual carbon rate is 24%, and T 5 =321°C, T 10 = 358°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com