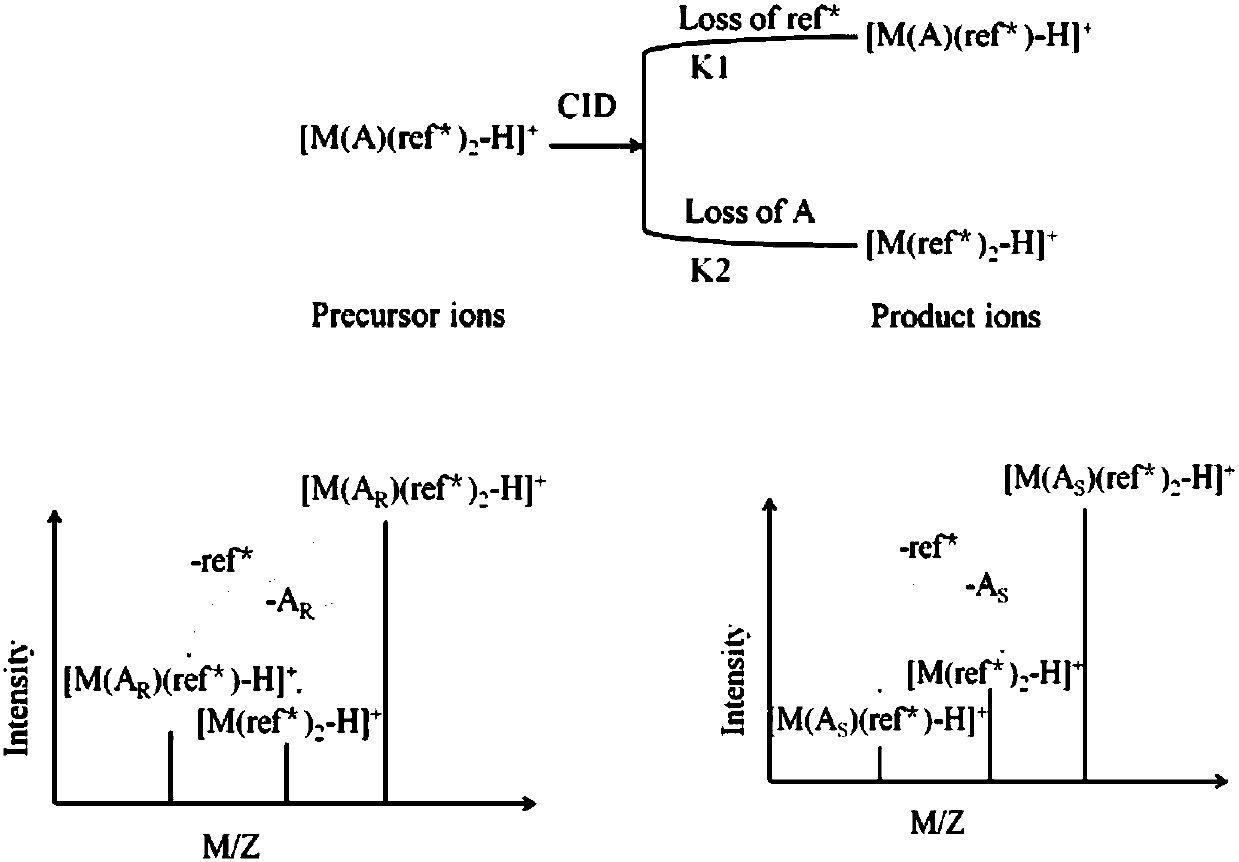
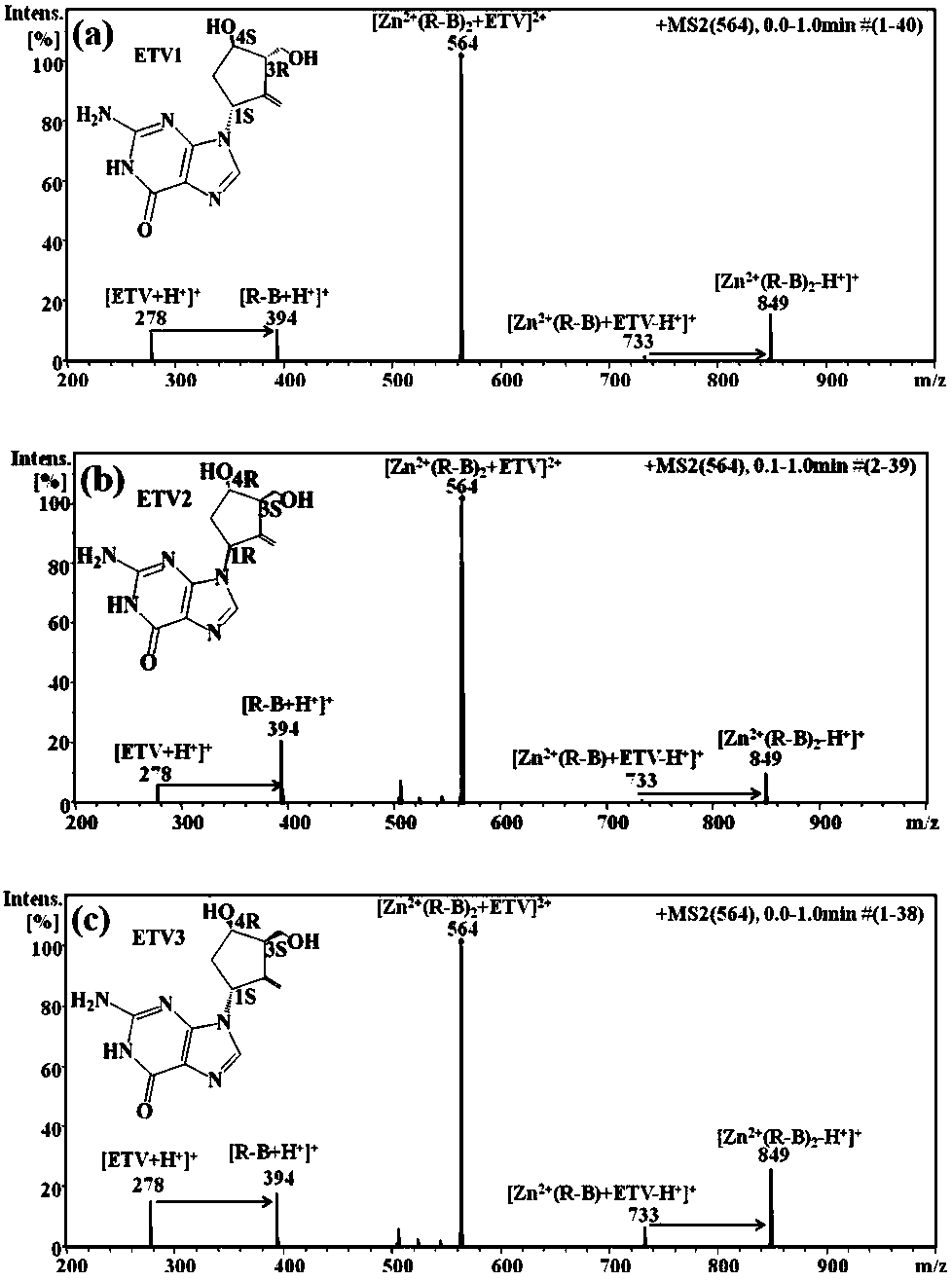
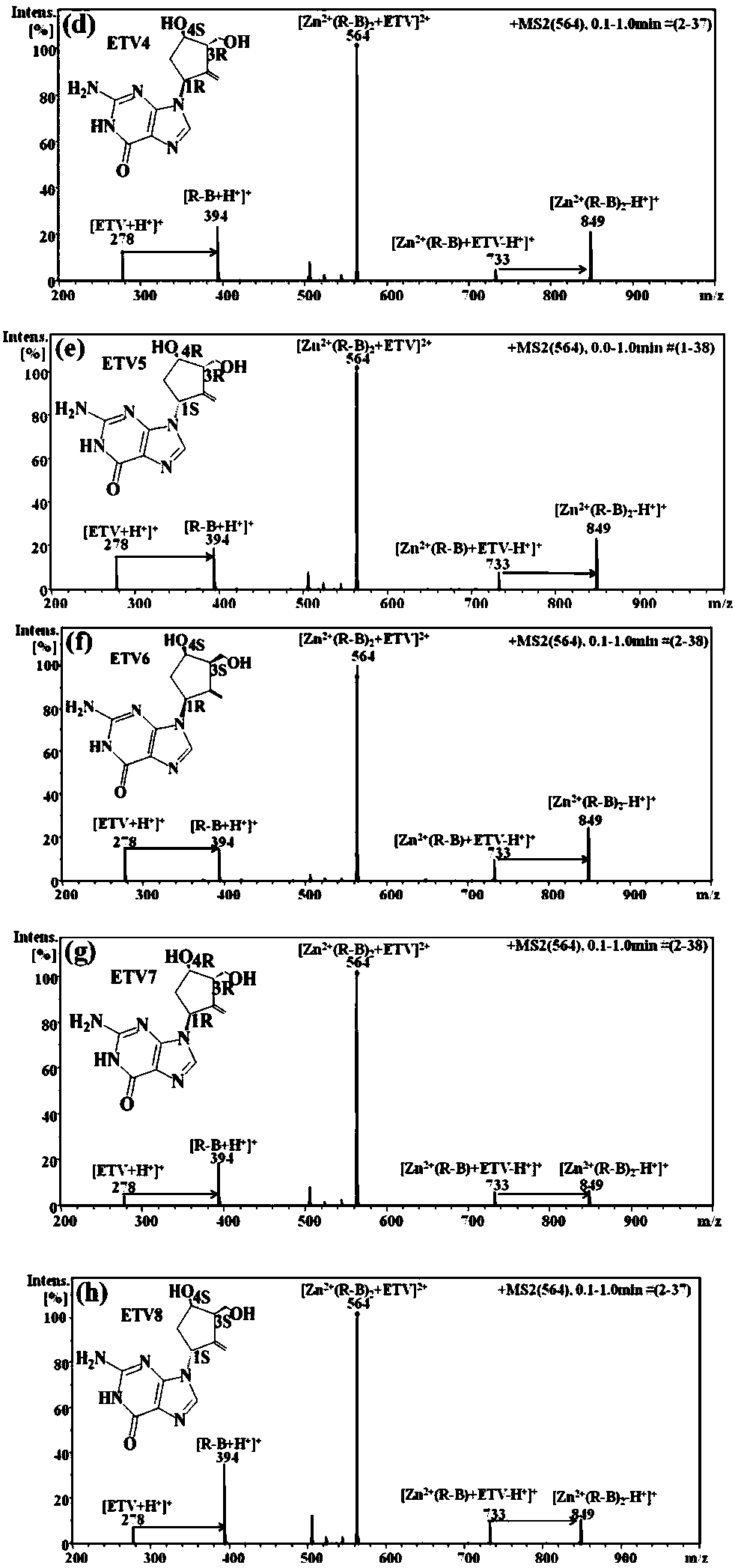
Distinguishing and determining method of entecavir chiral isomers
A technology for chiral isomers and entecavir, applied in the field of mass spectrometry analysis, can solve the problems of insufficient enantiomeric separation, long solvent gradient elution time, no simultaneous separation of chiral isomers, etc., so as to improve the separation efficiency. , method fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1) Analytical sample preparation: the pure products of each single configuration of entecavir are dissolved in 50v / v% methanol aqueous solution to prepare 100μg / ml standard solution for use, and R-besifloxacin powder is dissolved in methanol to 1mg / ml For use, metal salt ZnSO 4 The powder was dissolved and diluted in ultrapure water into a 1mg / ml stock solution for later use; the standard solutions of each configuration of entecavir were used with R-besifloxacin solution and ZnSO respectively 4 Solution mixing; final concentration of entecavir in each mixed solution is 25μg / ml, final concentration of R-besifloxacin is 25μg / ml, ZnSO 4 The final concentration is 2.5μg / ml.
[0031] 2) Determine the working conditions of the mass spectrometer: Bruker ion trap mass spectrometer is used, the electrospray ion source is selected, and the data is processed by Compass DataAnalysis software. Instrument parameter settings: spray needle voltage, -4500V; nitrogen as atomizing gas, pressu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com