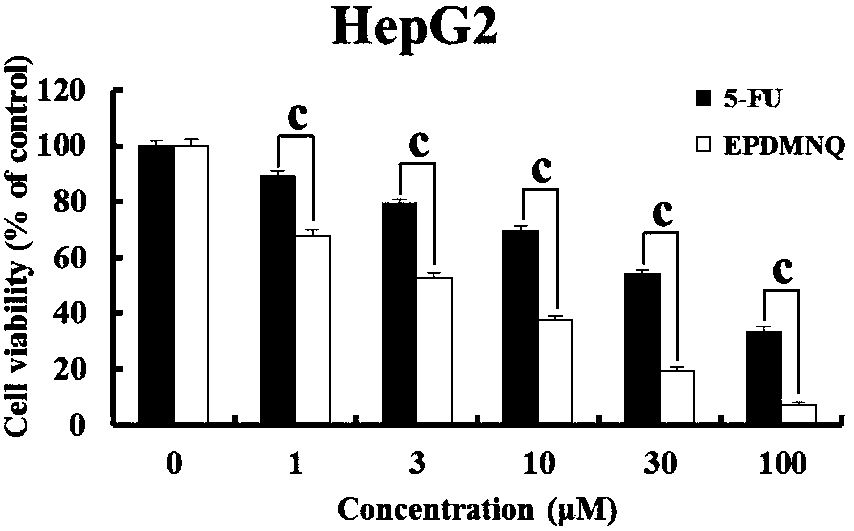
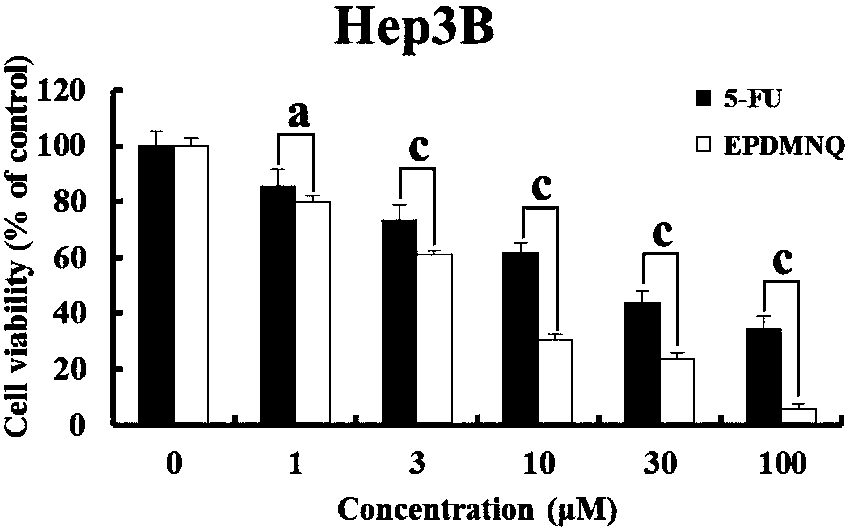
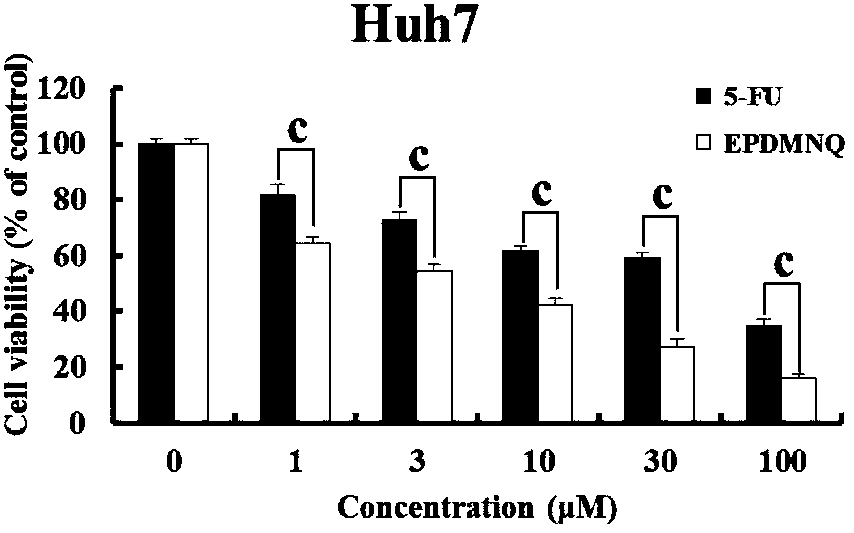
2,3-epoxy-2-propyl sulfone-5,8-dimethoxy-1,4-naphthoquinone as well as preparation method thereof, and medicine containing same
A dimethoxy, naphthoquinone technology, applied in the field of medicine, can solve the problems of liver function damage, blood picture reduction, lack of discrimination ability of chemotherapy, etc., and achieve the effect of reducing toxic side effects, low irritation and toxicity, and low normal cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0054] Example: Preparation of 2,3-epoxy-2-propylsulfone-5,8-dimethoxy-1,4-naphthoquinone compound in this example:
[0055] 1. Synthesis of 2-propylmercapto-5,8-dimethoxy-1,4-naphthoquinone
[0056] In a 100ml reaction bottle, add 218.21mg (1mmol) of 5,8-dimethoxy-1,4-naphthoquinone and 50ml of methanol, mix well, add 111.02μl (1.5mmol) of 1-propanethiol, and react at room temperature for 4 hours Finally, 149.8 mg (0.2 mmol) of sodium dichromate and 27.2 μl (0.75 mmol) of concentrated sulfuric acid were added to the mixture, and the reaction was completed after 5-10 minutes. Extracted with dichloromethane and saturated brine, dried with an appropriate amount of anhydrous sodium sulfate, filtered, and concentrated to dryness to obtain a crude product, which was prepared by column chromatography, 2-propylmercapto-5,8-dimethoxy-1,4- naphthoquinone.
[0057] 2. Synthesis of 2,3-epoxy-2-propylsulfone-5,8-dimethoxy-1,4-naphthoquinone (EPDMNQ)
[0058] In a 50ml reaction bottle, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com