Soluble microneedle system and application thereof
A soluble, micro-needle technology, applied in the field of medicine, can solve problems such as safety risks, achieve good safety performance, improve therapeutic effects, and improve cosmetic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of soluble microneedle system
[0036] Reverse mold preparation: mix the PDMS matrix and curing agent uniformly in a volume ratio of 10:1, leave it to stand to remove bubbles, pour it on the silicon microneedle, heat it on a microcomputer electric hot plate, and heat it at 80°C for 3 hours. It is cured until it is heated and solidified from a liquid with strong fluidity to a solid state, and then the film is removed to obtain a PDMS reversal mold with pinholes on the surface that matches the shape and number of the HA microneedle body.
[0037] Prepare polyvinylpyrrolidone (PVP) and / or hyaluronic acid (HA), as well as poloxamer 407 and carboxymethyl cellulose sodium (CMC-Na) three or four polymers according to the ratio shown in Table 1. The material mixture solution is injected into the PDMS reversal mold, the microneedle hole is filled to the full, placed in a closed container at 3 times the atmospheric pressure for 3 minutes, the filled microneedle m...
Embodiment 2
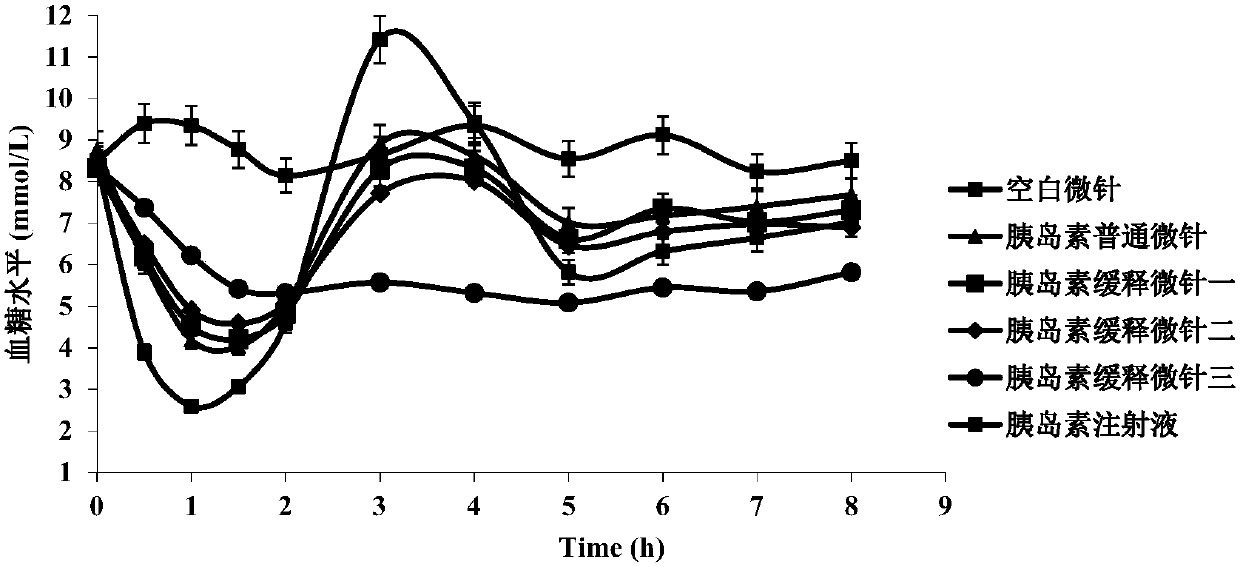
[0043] Example 2 Preparation of insulin-loaded soluble microneedles and comparison of sustained release effects
[0044] Reverse mold preparation: mix the PDMS matrix and curing agent uniformly in a volume ratio of 10:1, leave it to stand to remove bubbles, pour it on the silicon microneedle, heat it on a microcomputer electric hot plate, and heat it at 80°C for 3 hours. It is cured until it is heated and solidified from a liquid with strong fluidity to a solid state, and then the film is removed to obtain a PDMS reversal mold with pinholes on the surface that matches the shape and number of the HA microneedle body.
[0045] Add insulin powder to polyvinylpyrrolidone (PVP) and / or hyaluronic acid (HA), and a mixed solution of poloxamer 407 and carboxymethyl cellulose sodium (CMC-Na) three or four polymer materials (The weight percentages of these polymer materials in the mixed solution are shown in Table 1), inject the PDMS inversion mold, fill the micro-pinholes to full, and place ...
Embodiment 3
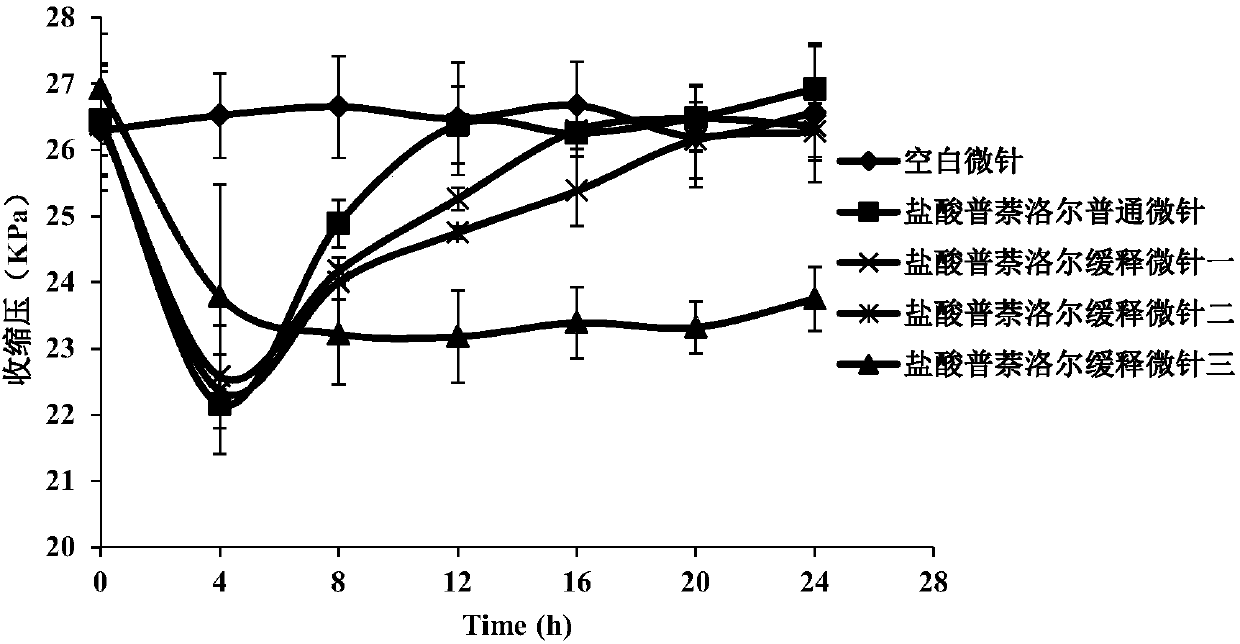
[0052] Example 3 Preparation of soluble microneedles containing propranolol hydrochloride and comparison of sustained-release effects
[0053] Add propranolol hydrochloride to polyvinylpyrrolidone (PVP) and / or hyaluronic acid (HA), as well as poloxamer 407 and carboxymethyl cellulose sodium (CMC-Na) three or four polymer materials In the mixed solution (the weight percentages of these polymer materials in the mixed solution are shown in Table 1), inject the PDMS inversion mold, fill the micro-pinholes to full, and place in a closed container at 3 times the atmospheric pressure for 3 minutes. The filled microneedle mold was placed in a desiccator to dry for 24 hours, and the microneedle was peeled from the PDMS reversal mold to obtain propranolol hydrochloride sustained-release microneedle III.
[0054] At the same time, use the same method to prepare propranolol hydrochloride microneedles for comparison:
[0055] 1. Add propranolol hydrochloride to PVP K30 Inject the PDMS reversal m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Height | aaaaa | aaaaa |
| Height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com