Preparation method of manganese zinc soft magnetic ferrite
A technology of manganese-zinc soft magnets and oxygen bodies, which is applied in the direction of magnetic materials, magnetic objects, and inorganic materials, can solve the problems of low saturation magnetic flux density and high core loss, achieve high saturation magnetic flux density, and improve saturation The effect of magnetic flux density and uniform fine particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
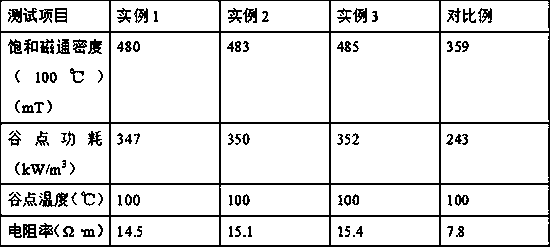
example 1
[0028] Put 100g pyrolusite ore in a pulverizer and pulverize for 3h, pass through a 100 mesh sieve to obtain sieved pyrolusite powder, place the pyrolusite powder in a conical flask, add 40g iron powder to the conical flask, 200mL mass fraction is 45 % sulfuric acid solution and 20g orange peel, after stirring and mixing for 20min, move the conical flask into a water bath, heat up to 50°C, keep warm for 2h, and obtain a leaching solution; add 70g manganese dioxide to the leaching solution, and heat up to 80°C , reacted for 2 hours to obtain the oxidized leaching solution, continue to add 30 g of manganese carbonate to the oxidized leaching solution, stir until no bubbles are produced, carry out suction filtration to the oxidizing leaching solution, remove the filter residue to obtain the filtrate, transfer the filtrate to a beaker, and add a mass fraction of 25% ammonia water, adjust the pH to 6.5, stir for 5 minutes, filter and separate under normal pressure to obtain a deep i...
example 2
[0030] 110g pyrolusite ore is placed in the pulverizer and pulverized for 3.5h, and passed through a 100 mesh sieve to obtain sieved pyrolusite powder, which is placed in a conical flask, and 45g iron powder, 250mL mass fraction is added to the conical flask. 45% sulfuric acid solution and 25g orange peel were stirred and mixed for 25min, then the conical flask was moved into a water bath, heated to 52°C, and kept for 2.5h to obtain a leaching solution; 75g of manganese dioxide was added to the leaching solution, and the temperature was raised to React for 2.5 hours at 85°C to obtain the oxidized leaching solution. Continue to add 35g of manganese carbonate to the oxidized leaching solution and stir until no bubbles are generated. Suction filter the oxidized leaching solution to remove the filter residue to obtain the filtrate. Transfer the filtrate to a beaker and add Ammonia water with a mass fraction of 25%, adjust the pH to 6.7, stir for 7 minutes, filter and separate under...
example 3
[0032] Put 120g pyrolusite ore in a pulverizer and pulverize it for 4h, pass through a 100 mesh sieve to obtain sieved pyrolusite powder, place the pyrolusite powder in a conical flask, add 50g iron powder to the conical flask, 300mL mass fraction is 45 % sulfuric acid solution and 30g orange peel, after stirring and mixing for 30min, move the conical flask into a water bath, heat up to 55°C, keep warm for 3h, and obtain a leaching solution; add 80g of manganese dioxide to the leaching solution, and heat up to 90°C , reacted for 3h to obtain the oxidized leachate, continue to add 40g of manganese carbonate to the oxidized leachate, stir until no bubbles are produced, carry out suction filtration to the oxidized leachate, remove the filter residue to obtain the filtrate, transfer the filtrate to a beaker, add a mass fraction of 25% ammonia water, adjust the pH to 7.0, stir for 10 minutes, filter and separate under normal pressure to obtain a deep iron removal solution; add 120mL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com